Translate this page into:
Sonographic Features of Diffuse Hashimoto Thyroiditis: Determining Sensitivity of Features and Predictors of Malignancy

Corresponding Author: Swapnil Patel, Department of Imaging Sciences, University of Rochester Medical Center, 601 Elmwood Ave, PO Box 648, Rochester, New York, United States. E-mail: swapnil_patel@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Patel S, Giampoli E, Oppenheimer D, Montoya S, Rupasov A, Dogra V. Sonographic Features of Diffuse Hashimoto Thyroiditis: Determining Sensitivity of Features and Predictors of Malignancy. Am J Sonogr 2018, 1(6) 1-7
Abstract
Objective
The objective of this study was to determine the sensitivity and accuracy of five sonographic features of Hashimoto thyroiditis (HT) in histologically confirmed cases. In addition, to determine if sonographic features can serve as a predictor of underlying malignancy.
Methods
A cohort of 101 patients who underwent thyroidectomy for malignancy or other clinical conditions with histologically confirmed HT and a pre-operative thyroid ultrasound examination were included. A retrospective analysis of sonographic features and radiology reports was conducted and comparative statistical analysis completed.
Results
Among the cohort of 101 patients, 84% demonstrated sonographic evidence of HT. The following features were used for the identification of HT: Diffuse hypoechoic echogenicity, parenchymal heterogeneity, hypoechoic micronodularity, echogenic septations, and hypervascularity. There was a female predominance (9:1) with an average age of 50 years. Among the studied sonographic features of HT, parenchymal heterogeneity was the most sensitive (88.2%) and hypervascularity was the least sensitive (17.7%). Approximately 44% of the study cohort had malignancy; papillary thyroid carcinoma was by far the most common, accounting for 89% of all malignancies. Hypoechoic micronodularity was the feature with the greatest positive predictive value (PPV) for malignancy. Nodal metastasis was less common in patients with sonographically evident HT.
Conclusion
HT is the most common cause of hypothyroidism in iodine sufficient areas of the world. Parenchymal heterogeneity and diffuse hypoechogenicity were the most sensitive sonographic features of HT. Hypoechoic micronodularity demonstrated the greatest PPV for malignancy.
Keywords
Cancer
Hashimoto thyroiditis
Thyroid gland
Ultrasound
INTRODUCTION
Hashimoto thyroiditis (HT) is the most common type of inflammatory thyroid disease and the most frequent cause of hypothyroidism in iodine sufficient areas of the world including the United States.[1] Development of antithyroid antibodies is the driving pathophysiologic process resulting in the lymphocytic infiltrative changes that are the hallmark of HT, most specifically elevated levels of antithyroid peroxidase (TPO) and antithyroglobulin antibodies, which are seen in 90% and up to 50% of HT patients, respectively.[2]Clinically, patients are hypothyroid and depending on the chronicity, can manifest as diffuse goiter, normal sized, or atrophic thyroid gland. HT may be indolent, and therefore, subclinical disease can have adverse effects on lipid metabolism and cardiovascular health in the absence of symptoms.[3] Sonographic evaluation of the thyroid combined with serologic measurements can improve detection and facilitate early treatment of HT. Sonographically, numerous imaging features have been described with HT including diffusely hypoechogenicity, coarse and heterogeneous parenchymal echotexture, echogenic septations, hypoechoic micronodularity, and hypervascularity.[4] There is a known association of thyroid cancer in patients with HT, specifically papillary thyroid carcinoma (PTC) and lymphoma.[5] The purpose of this study was to determine which sonographic features are most sensitive for HT and the presence of underlying malignancy.
METHODS
Subjects
Patients at a single U.S. medical center were included in this IRB-approved study. All patients underwent total or subtotal thyroidectomy between January 2011 and June 2015 and had a pre-operative diagnostic thyroid ultrasound examination within 1 year before surgery. Patient demographics were obtained from the radiology and/or pathology reports including age and gender.
Pathologic analysis
Patients who had undergone complete or partial thyroidectomy, most commonly for diffuse thyroid disease (20/101), nodule or mass (65/101), toxic and non-toxic nodular goiter (10/101), hyperparathyroidism (3/101), or other cervical findings suspicious for malignancy (3/101) were included in the study if there was histopathologic evidence of HT/chronic lymphocytic thyroiditis. Surgical histopathology reports were reviewed to determine the presence and extent of malignancy.
Sonographic analysis
Pre-operative ultrasound examinations were performed by sonographers on staff at a single tertiary level hospital using 10 MHz or higher linear probe (Logiq 9, GE, Waukesha, WI, USA). These examinations were retrospectively reviewed by a radiologist, and five predetermined sonographic features were analyzed for evaluation of diffuse form of HT. Specifically, the thyroid parenchyma was evaluated for the presence or absence of diffuse hypoechoic echogenicity (defined as diffuse decreased parenchymal echogenicity compared with adjacent strap muscles), parenchymal heterogeneity (defined as coarse echotexture with areas of mixed echogenicity involving >50% gland volume), echogenic septations (defined as diffuse or geographic regions of linear echogenic bands), hypoechoic micronodularity (defined as diffuse pattern of numerous hypoechoic nodules up to 6 mm), and hypervascularity (Figures 1-5). Color Doppler examination of whole gland vascularity was performed utilizing pulse repetition frequency and color Doppler gain levels optimized to minimize aliasing in carotid artery and internal jugular vein. Vascularity was assessed based on visual scale per classification described by Schulz et al. with types 1–3 representative of variable hypervascular state (Table 1).[6] All examinations were peer reviewed by a second radiologist and a standardized interpretation was established to assess and minimize interobserver variability. Sonographically, the presence of HT was defined as the presence of one or more of the five sonographic characteristics. Final dictated radiology reports of the examinations were reviewed by an investigator blinded to the sonographic analysis to assess for diagnostic concordance.
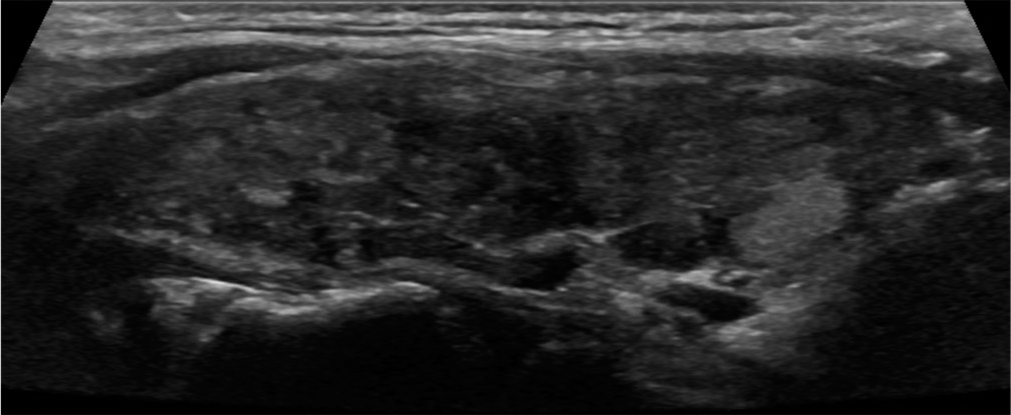
- Parenchymal heterogeneity. A 37-year-old female with chronic lymphocytic (Hashimoto) thyroiditis presented with enlarging thyroid nodule. Sagittal gray scale ultrasound image of the thyroid gland demonstrates diffusely heterogeneous echotexture.
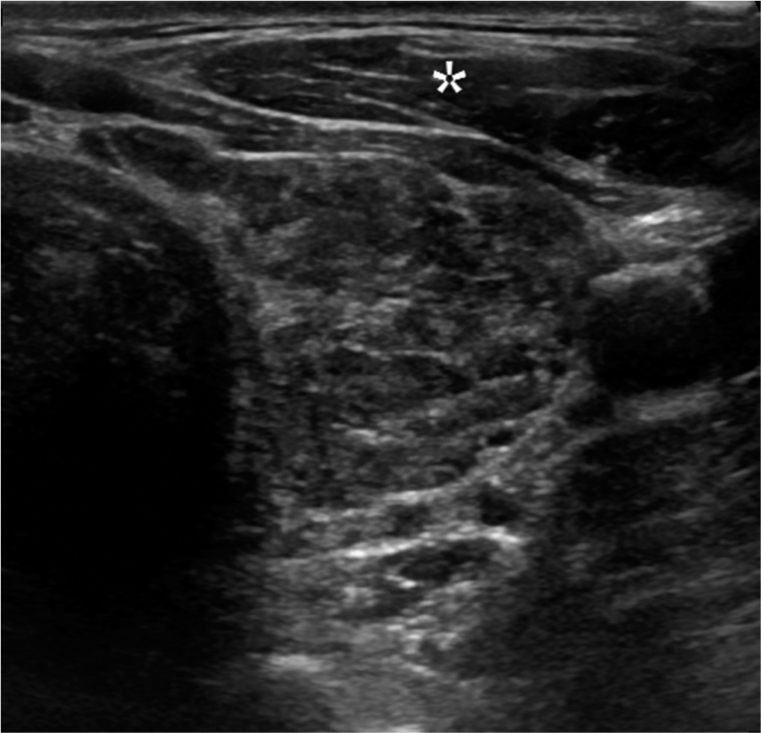
- Hypoechogenicity. A 35-year-old female with Hashimoto thyroiditis presented with thyroid mass. Transverse gray scale ultrasound image of the thyroid gland illustrates the parenchyma is iso- to hypoechoic to the adjacent strap musculature (*).

- Hypoechoic micronodularity. A 49-year-old female with Hashimoto thyroiditis presented with neck fullness. Sagittal gray scale ultrasound image of the thyroid gland with diffusely scattered hypoechoic micronodules.

- Echogenic septations. A 45-year-old female with Hashimoto thyroiditis presented for evaluation of hypothyroidism. Sagittal gray scale ultrasound image of the thyroid gland reveals scattered hyperechoic linear bands throughout the parenchyma (arrow).

- Hypervascularity. A 57-year-old female with Hashimoto thyroiditis presented for follow-up evaluation of indeterminate thyroid nodule. Transverse color Doppler ultrasound image of the thyroid gland demonstrates diffusely increased vascularity throughout the parenchyma. Common carotid artery and internal jugular vein are seen adjacent to the thyroid gland.
| Pattern type | Color Doppler appearance |
|---|---|
| 0 | Parenchymal flow is minimal or absent. Visualized flow limited to major peripheral vessels |
| 1 | Mildly increased parenchymal flow |
| 2 | Clearly increased parenchymal flow with homogenous distribution |
| 3 | Markedly increased parenchymal flow with homogenous distribution including “thyroid inferno” appearance |
Statistical analysis
Mann–Whitney test was used in comparison of continuous variables and the Fisher’s exact test for the comparison of categorical variables. Regression analysis performed with Spearman correlation coefficients. Values are provided either as mean ± standard deviation (range) or percentage (95% confidence interval). In all analyses, P < 0.05 was taken to indicate statistical significance.
RESULTS
A total of 101 patients who had undergone thyroidectomy with histological evidence of HT and a pre-operative thyroid ultrasound examination were identified. In this study cohort, about 91% were women (92/101) and the average age was 51 years old (Table 2). 85 patients demonstrated at least one of the five sonographic features of HT with an interobserver agreement of 84.4% (kappa score of 0.54). Among the five sonographic features of HT studied, parenchymal heterogeneity demonstrated the highest sensitivity (88.2%). Hypervascularity was the least frequently encountered feature, observed in 16 patients (10 patients with type 1, 5 patients with type 2, 1 patient with type 3) with a sensitivity of 17.7% (Table 3). The presence of both diffuse hypoechogenicity and parenchymal heterogeneity correlated the best with HT with r = 0.39 (Table 4). The presence of HT was mentioned in radiology report in greatest frequency when three or more sonographic features were present (Table 5).
| Characteristic | Total | Sonographic features of HT | No sonographic features of HT | p-value |
|---|---|---|---|---|
| Patients, n (%) | 101 | 85 (84) | 16 (16) | |
| Mean age, years (range) | 50.2 (11–80) | 49.9 (11–79) | 52.1 (20–80) | 0.61 |
| Male, n (%) | 9 (9) | 9 (11) | 0 (0%) | 0.35 |
| Female, n (%) | 92 (91) | 76 (89) | 16 (100) | 0.83 |
HT: Hashimoto thyroiditis
| Sonographic feature | Sensitivity (%) | p-value |
|---|---|---|
| Diffusely hypoechoic echogenicity | 48.2 (37.3–59.3) | 0.0001 |
| Parenchymal heterogeneity | 88.2 (79.4–94.2) | <0.0001 |
| Echogenic septations | 18.8 (11.2–28.8) | 0.07 |
| Hypoechoic micronodules | 67.1 (56.0–76.9) | <0.0001 |
| Hypervascularity (types 1–3) | 17.7 (10.2–27.4) | 0.12 |
Values in parentheses are 95% confidence intervals, HT: Hashimoto thyroiditis, p-value generated using Fisher’s exact test
| Sonographic Features | Diffusely hypoechoic echogenicity | Parenchymal heterogeneity | Echogenic septations | Hypoechoic micronodules | Hypervascularity |
|---|---|---|---|---|---|
| Diffusely hypoechoic echogenicity | 0.39 (p<0.0001) | 0.25 (p=0.01) | 0.24 (p=0.02) | 0.17 (p=0.1) | |
| Parenchymal heterogeneity | 0.39 (p<0.0001) | 0.26 (p=0.01) | 0.26 (p=0.009) | 0.182 (p=0.07) | |
| Echogenic septations | 0.25 (p=0.01) | 0.26 (p=0.01) | 0.05 (p=0.6) | −0.03 (p=0.8) | |
| Hypoechoic micronodules | 0.24 (p=0.02) | 0.26 (p=0.009) | 0.05 (p=0.6) | 0.03 (p=0.8) | |
| Hypervascularity | 0.17 (p=0.1) | 0.18 (p=0.07) | −0.03 (p=0.8) | 0.03 (p=0.8) |
Values represent Spearman correlation coefficients, with p-values in parentheses, HT: Hashimoto thyroiditis
| Number of sonographic features present | Total | HT included in radiology report | HT not included in radiology report | p-value |
|---|---|---|---|---|
| None | 16 | 2 | 14 | <0.01 |
| One | 18 | 6 | 12 | 0.07 |
| Two | 21 | 11 | 10 | 0.99 |
| Three | 34 | 26 | 8 | <0.01 |
| Four | 10 | 8 | 2 | 0.11 |
| Five | 2 | 2 | 0 | 0.5 |
P value is result of Fisher’s exact test. HT: Hashimoto thyroiditis
Approximately 44% (45/101) of the study cohort had malignancy with PTC comprising 89% (40/45) of malignancies. The remainder included three cases of follicular carcinoma, one case of anaplastic carcinoma, and one case of lymphoma. Of the 45 malignancies, 16 had documented nodal metastasis with a statistically significant larger proportion occurring in patients without any sonographic features of HT (Table 6). No statistically significant difference was observed between the age and gender of patients with malignancy compared with those without malignancy. There was no significant correlation between sonographic evidence of HT and incidence of malignancy (Table 6).
| Sonographic features of HT n=85 | No sonographic features of HT n=16 | p-value | |
|---|---|---|---|
| Mean age (range) | 49.9 (11–79) | 52.1 (20–80) | 0.61b |
| Malignancy, n (%) | 39 (46%) | 6 (38%) | 0.59a |
| Type of malignancy | |||
| Papillary (PTC) | 35 (41%) | 5 (31%) | 0.79a |
| Follicular | 3 (4%) | 0 (0) | |
| Anaplastic | 0 (0) | 1 (6%) | |
| Lymphoma | 1 (1%) | 0 (0) | |
| Nodal metastasis (% positive) | 11 (28%) | 5 (83%) | 0.04a |
Values are provided either as mean (range) or percentage (95% confidence interval), HT: Hashimoto thyroiditis, p-values generated as follows: a=Fisher’s exact test, b=Mann–Whitney test
Parenchymal heterogeneity and hypoechoic micronodularity were more common in patients with HT and malignancy compared to patients with HT and no malignancy, with sensitivities of 71% and 66%, respectively. Of the five studied sonographic features, hypoechoic micronodularity was most commonly present with malignancy (Table 7).
| Feature | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | p-value |
|---|---|---|---|---|---|
| Diffusely hypoechoic echogenicity | 46.7 (31.7–62.1) | 64.3% (50.4–76.6) | 51.2 (35.1–67.1) | 60.0 (46.5–72.4) | 0.31 |
| Parenchymal heterogeneity | 71.1 (55.7–83.6) | 23.2 (13.0–36.4) | 42.7 (31.3–54.6) | 50.0 (29.9–70.1) | 0.65 |
| Echogenic septations | 13.3 (5.1–26.8) | 82.1 (69.6–91.1) | 37.5 (15.2–64.6) | 54.1 (43.0–65.0) | 0.59 |
| Hypoechoic micronodules | 66.7 (51.1–80.0) | 51.8 (38.0–65.3) | 53.6 (39.0–66.0) | 65.9 (50.1–79.5) | 0.07 |
| Hypervascularity (types 1–3) | 17.8 (8.0–32.1) | 87.5 (75.9–94.8) | 53.3 (26.6–78.7) | 57.0 (45.9–67.6) | 0.58 |
Values in parentheses are 95% confidence intervals. p-value is result of Fisher’s exact test
DISCUSSION
HT is the leading cause of hypothyroidism in the US activated by thyroid antigens, T- and B-lymphocytes drive immune-mediated infiltration, and destruction of the thyroid parenchyma. On histopathological examination, this inflammatory process produces lymphocytic aggregates, shrunken thyroid follicles, and varying degree of fibrosis.[7] A genetic predisposition has been postulated, which may be triggered by environmental factors. This theory is supported by the fact that patients with HT also have other forms of autoimmune disease. HT most commonly occurs in females between 30 and 50 years of age. The diagnosis is strongly supported by low serum T4 levels, elevated TSH levels, and presence of antithyroid antibodies, specifically anti-TPO antibodies. However, up to 30% of HT patients exhibit no or low autoantibodies and 2–20% of the unaffected population may have autoantibodies, which make diagnosis of HT challenging on the basis of serologic tests alone.[8] Sonographic evaluation of the thyroid gland, in combination with serologic and clinical assessments, improves diagnostic accuracy. Some studies including a 482 patient cohort study Rago et al. showed the diagnostic utility of ultrasound in prediction of current and future hypothyroidism among asymptomatic patients surpassed that of autoantibodies.[9] Histologic evaluation remains the gold standard in confirming the presence of HT.
Sonographic features of diffuse HT
Well-recognized sonographic features of diffuse HT include a hypoechoic and enlarged thyroid gland with heterogeneous parenchyma containing echogenic septations and micronodules.[8] Overall, the present study demonstrated 84% sensitivity in detection of HT sonographically. Parenchymal heterogeneity was found to be most sensitive finding in HT followed by hypoechoic micronodularity and diffuse hypoechogenicity. These findings are in agreement with previously published studies with diffusely hypoechoic appearance of the thyroid gland as an indicator of hypothyroidism with a positive predictive value (PPV) up to 88%.[10] Hypoechoic micronodularity is also recognized as a key diagnostic feature with a PPV up to 95%.[8,11] These two features are believed to represent manifestations of the diffuse lymphocytic infiltration of the thyroid gland over time. Parenchymal heterogeneity and diffuse hypoechogenicity are not only suggestive of HT but also are postulated to be correlated with the degree of immune-mediated changes and extent of hypothyroidism.[10]
In addition, the other sonographic features, despite lacking strong predictive values, also serve as markers of disease. In the present study, hypervascularity was the least commonly observed feature as it was present in only 18% of HT patients. This may reflect a greater proportion of patients with chronic (burned out) disease. Hypervascularity exists on a continuum related to the stage of autoimmune thyroid disease with most marked vascularity during the initial inflammatory stage which tapers off in the later fibrotic stage.[12,13] The hypervascularity during the initial inflammatory stage heralds the development of varying degrees of hypothyroidism as blood flow is recruited by elevated TSH levels in response to countless cycles of follicular damage and scarring, which eventually lead to a chronic avascular state.[8,12] Overall, it is understood that the co-occurrence of several sonographic features of HT improves sensitivity and accuracy in diagnosis.[14] Review of the radiology reports demonstrated diagnostic confidence was best when three or more sonographic features were present.
HT and coexisting thyroid malignancy
Malignancy can occur in patients with HT, with some authors reporting a significant increased risk above the average population. For example, a nationwide cohort study conducted in Taiwan demonstrated a 1.68-fold greater risk of developing malignancy in patients with HT.[15] Specifically, primary thyroid lymphoma has been classically associated with HT; however, it is rare relative to PTC.[5] Although not confirmed, a number of pathophysiological mechanisms have been proposed to explain these observations including TSH and immunologic driven malignant degeneration of chronic thyroiditis and increased expression of oncogenes in HT patients.[16] Furthermore, increased risk of colon, lung, and breast cancer has been reported in HT patients, related to underlying autoimmune processes.[15] The present study compared the occurrence of thyroid malignancy in patients who had histologically confirmed HT with and without sonographically evident HT. Malignancy occurred in slightly higher proportion in patients with sonographic evidence of HT compared to those without the sonographic features of HT, although this was not statistically significant. Conversely, about 87% of patients with PTC (the most commonly observed malignancy) also had sonographic evidence of HT. There was no significant trend among patient demographic factors and the incidence of malignancy although other studies have noted malignancy more frequently occurred in the young and middle age groups with female predominance.[13,15]
Although the risk of malignancy in HT is increasingly scrutinized in the literature, relatively few studies have investigated the specific sonographic features of HT that are correlative with malignancy. This was one of the goals of the present study. While none of the sonographic features reached statistical significance, parenchymal heterogeneity was the most sensitive feature, while hypoechoic micronodularity demonstrated the greatest PPV for malignancy (Table 5). This may be linked to the aforementioned concept that these two sonographic features are associated with a more advanced stage of HT. It has been shown that nodular form of HT is more frequently encountered in the thyroid gland demonstrating a background of diffuse HT than without diffuse HT.[17] It can be postulated that this combination of nodular pattern, parenchymal heterogeneity and hypoechoic micronodularity may be interlinked and associated with an advanced disease stage and theoretically an increased risk of malignancy.
In concordance with previous work, nodal metastases were more common in patients without sonographic findings of HT, occurring in about 83%. This corroborates the general understanding in the literature that patients with thyroid malignancy and HT have lower rates of capsular invasion and extrathyroidal extension and therefore a more favorable prognosis.[18] This finding suggests that patients without sonographic evidence of HT may have an earlier and less sonographically evident stage of thyroid disease and thus are not yet afforded this protective effect.
The limitations of this study include the modest sample size which comprised patients from a single institution, which may not be entirely representative of the general population and limit the statistical power to analyze significant differences between the subgroups. In addition, there is potential selection bias as our patients were recruited from a pool of patients with clinically evident thyroid abnormalities which were ultimately treated surgically. Therefore, our cohort likely included patients with more advanced stages of thyroid disease.
CONCLUSION
The diagnostic accuracy of HT is improved when combining sonographic evaluation to the established clinical and serologic parameters, leading to improved recognition of disease, which is particularly useful in asymptomatic and euthyroid individuals. Parenchymal heterogeneity is the most sensitive finding in HT followed by hypoechoic micronodularity and diffuses hypoechogenicity. Malignancy is known to occur in patients with HT, and hypoechoic micronodularity is the feature with the greatest PPV for malignancy. Nodal metastasis was more common in patients without sonographic features of HT.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vikram Dogra is on the Editorial Board of the Journal.
References
- The epidemiology of thyroid disease. Br Med Bull. 2011;99:39-51.
- [CrossRef] [PubMed] [Google Scholar]
- Using acoustic structure quantification during b-mode sonography for evaluation of Hashimoto Thyroiditis. J Ultrasound Med. 2015;34:2237-43.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography of Hashimoto’s thyroiditis. J Clin Ultrasound. 1986;14:123-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic appearance of thyroid cancer in patients with Hashimoto Thyroiditis. J Ultrasound Med. 2015;34:697-704.
- [CrossRef] [PubMed] [Google Scholar]
- Color Doppler sonography in hypothyroidism. Eur J Ultrasound. 2003;16:183-9.
- [CrossRef] [Google Scholar]
- Pathogenesis of Hashimoto’s Thyroiditis (chronic autoimmune thyroiditis) 2016. Available from http://www.uptodate.com. [Last accessed on 2017 Jun 26]
- [Google Scholar]
- Hashimoto thyroiditis: Part 1, sonographic analysis of the nodular form of Hashimoto Thyroiditis. AJR Am J Roentgenol. 2010;195:208-15.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dysfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24:763-9.
- [CrossRef] [PubMed] [Google Scholar]
- The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000;10:251-9.
- [CrossRef] [PubMed] [Google Scholar]
- Micronodulation: Ultrasonographic sign of Hashimoto Thyroiditis. J Ultrasound Med. 1996;15:813-9.
- [CrossRef] [PubMed] [Google Scholar]
- Roles of ultrasound and power doppler ultrasound for diagnosis of Hashimoto Thyroiditis in anti-thyroid marker-positive euthyroid subjects. Quant Imaging Med Surg. 2014;4:232-8.
- [Google Scholar]
- Clinical relationship between Hashimoto’s Thyroiditis and papillary thyroid cancer. Acta Oncol. 2011;50:1228-34.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic differentiation of asymptomatic diffuse thyroid disease from normal thyroid: A prospective study. AJNR Am J Neuroradiol. 2010;31:1956-60.
- [CrossRef] [PubMed] [Google Scholar]
- Cancer risk in patients with Hashimoto’s Thyroiditis: A nationwide cohort study. Br J Cancer. 2013;109:2496-501.
- [CrossRef] [PubMed] [Google Scholar]
- Increased incidence of well-differentiated thyroid cancer associated with Hashimoto Thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204:764-73.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographic features of nodular Hashimoto Thyroiditis. Ultrasound Q. 2016;32:271-6.
- [CrossRef] [PubMed] [Google Scholar]
- Thyroidectomy for Hashimoto’s Thyroiditis: Complications and associated cancers. Thyroid. 2008;18:729-34.
- [CrossRef] [PubMed] [Google Scholar]







