Translate this page into:
Sonographic Spectrum of Testicular Adrenal Rest Tumors

Corresponding Author: Zachary Nuffer, Department of Imaging Sciences, University of Rochester Medical Center, 601 Elmwood Ave, Box 648, Rochester, New York, 14642, United States. E-mail: zachary_ nuffer@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Nuffer Z, Lu M, Jefferson J, Dogra V. Sonographic Spectrum of Testicular Adrenal Rest Tumors. Am J Sonogr 2018, 1(15) 1-4.
Abstract
Testicular adrenal rest tumors (TARTs) are benign testicular masses but can lead to infertility without medical attention. It is important to identify TARTs in childhood, as early diagnosis has been shown to have good success in preserving fertility. It is also important to differentiate TARTs from other testicular masses to avoid unnecessary orchiectomy. Ultrasound is the preferred imaging modality for the evaluation of TARTs; however, sonographic differentiation from testicular neoplasms can sometimes be very difficult. In this article, we review the spectrum of sonographic features of TARTs and propose a decision tree that relies on these features, with the goal of increasing clinician’s confidence in diagnosing TARTs.
Keywords
Congenital adrenal hyperplasia
Testicular adrenal rest
Testicular mass
Ultrasound
INTRODUCTION
Testicular adrenal rest tumors (TARTs) are a type of testicular neoplasm that is commonly associated with congenital adrenal hyperplasia (CAH). First described in 1940 by Wilkins et al., TARTs are benign but may cause infertility without medical attention. The pathophysiology has yet to be fully elucidated; however, it is known that in some normal neonates adrenal tissue remnants remain in the testes—the result of aberrant adrenal cortical tissue that adhered to the gonads and descended during prenatal life.[1] It is hypothesized that increased adrenal corticotropic hormone (ACTH), as seen in CAH, may block regression and promote the growth of ectopic adrenal tissue.[2] Providing support for this hypothesis is the fact that TARTs are seen in other syndromes with chronically elevated ACTH, such as Nelson’s and Addison’s syndromes. It is important to identify TARTs early to avoid tumor growth, which leads to compression of the seminal tubules, causing oligospermatogenesis or azoospermia, potentially resulting in infertility.[3]
Diagnosis of TARTs requires imaging evaluation. In one case series, Avila et al. found that 42% of patients with CAH had a testicular mass or masses detected by radiologic examination.[3] Due to the difficulty palpating tumors within the rete testes, TARTs that are <2 cm in diameter are generally impalpable. Although both ultrasound and magnetic resonance imaging (MRI) are proven methods of diagnosis, ultrasound is preferred due to greater accessibility, lower cost, and higher sensitivity in the detection of tumors. MRI is best reserved for imaging the extent of disease, especially as part of a pre-operative evaluation for incompletely descended testes.
A retrospective study by Wang et al. found that the most common sonographic appearance of TARTs is bilateral hypoechoic masses near the mediastinum testes.[4] Further studies have shown that very small adrenal rests cannot be accurately detected on imaging, while the smallest visible tumors typically appear as hypoechoic lesions. Larger tumors may contain fibrous strands which can be visible as hyperechoic reflections, producing a more heterogeneous appearance (Figure 1).[5] MRI can be used to confirm the diagnosis when necessary (Figure 2).
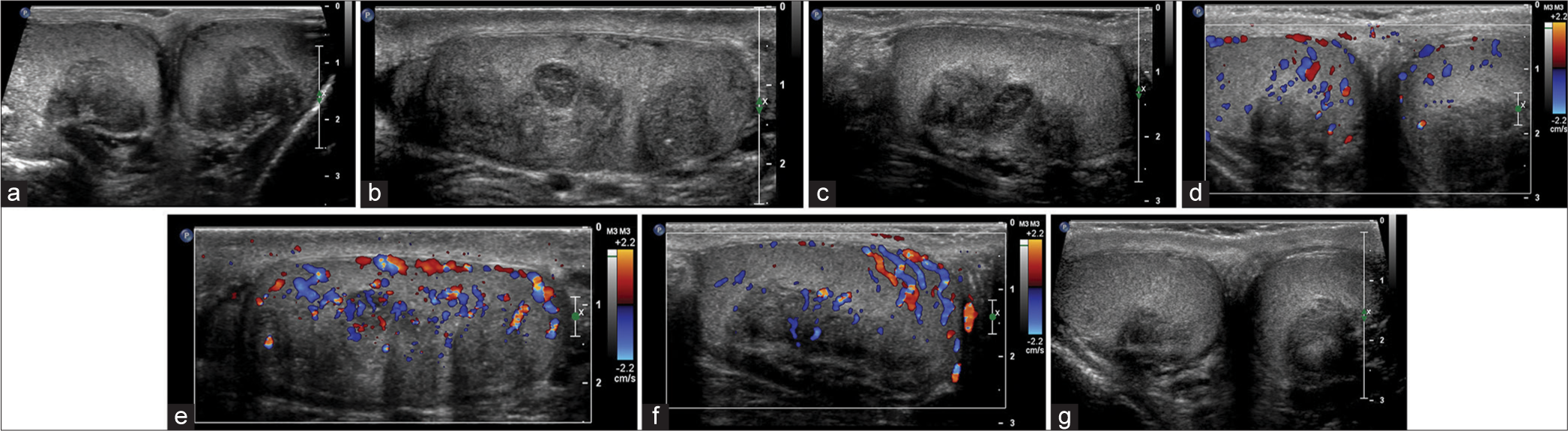
- 29-year-old man with congenital adrenal hyperplasia (CAH) and testicular masses consistent with bilateral testicular adrenal rest tumors. (a) Grayscale ultrasound “buddyshot” demonstrates large, lobulated, mediastinal testes masses with heterogeneous echogenicity, (b) Sagittal view of the extent of disease in the left testis, (c) Sagittal view of the extent of disease in the right testis, (d) color Doppler demonstrates scant vascular flow, (e) Sagittal view of the extent of blood flow in the left testis, (f) Sagittal view of the extent of blood flow in the right testis, (g) patient underwent treatment for CAH, following treatment the testicular masses have decreased significantly in size.
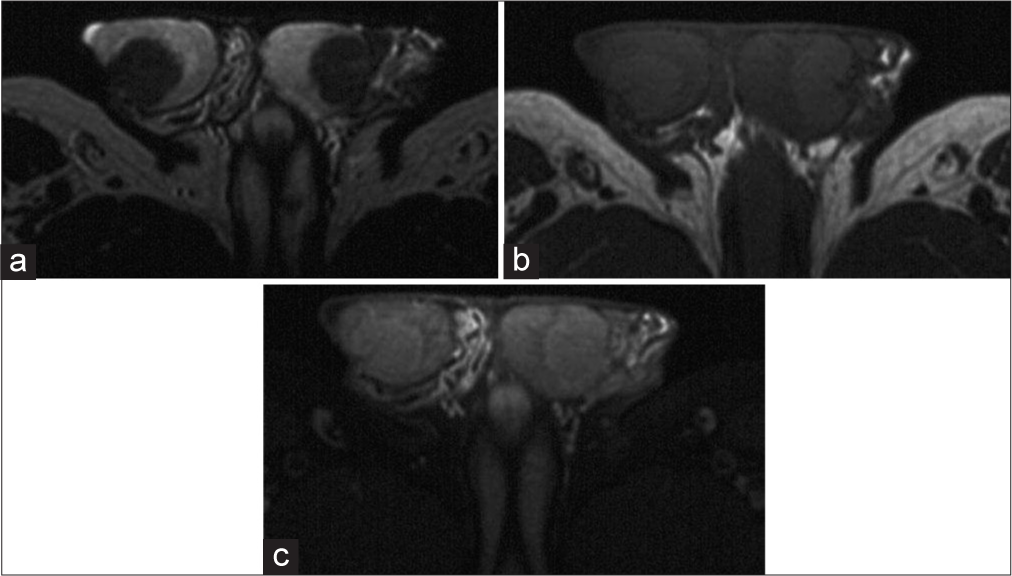
- 30 years old with bilateral testicular masses consistent with testicular adrenal rest tumors. (a) T2-weighted magnetic resonance imaging demonstrates bilateral hypointense masses at the mediastinum testis, (b) On pre-contrast T1 images, the masses are isointense to the surrounding testicular parenchyma, (c) On post-contrast T1 imaging, the masses enhance to the same extent as the surrounding testicular parenchyma.
As TARTs are benign with no malignant potential, they do not need to be removed at an early age. However, due to the central localization of the tumors near the mediastinum testis, long-term compression of the seminiferous tubules may lead to obstructive azoospermia and irreversible damage to the adjacent testicular tissue.[6,7] A recent study in individuals with CAH and bilateral TARTs found decreased seminiferous tubular diameter and varying degrees of peritubular fibrosis and hyalinization. In addition, there is evidence that patients with TARTs have a decreased number of germ cells. The irreversible consequence of hyalinization with obstruction of the lumen results in complete loss of germ cells and Sertoli cells, as well as a reduced number of Leydig cells.[8] For this reason, it is important to identify TARTs early and to distinguish them from other tumors of the testis, as radiographic diagnosis in childhood has been shown to have good success in preserving fertility. In this article, we review the ultrasound appearance of TARTs, focusing on sonographic features, echotexture, Doppler flow, location, border, and calcification.
IMAGING
Ultrasound findings of TARTs can be variable, but the most cited imaging characteristics include multiple hypoechoic, centrally located, lobulated masses, with minimal architectural distortion. Doppler flow evaluation usually demonstrates absent vascularity. Differentiating TARTs from other pathologies, including non-germ cell tumors or lymphoma, can sometimes be difficult. Lymphoma tends to appear as multiple hypoechoic lobulated lesions with increased vascularity on Doppler.[9] Furthermore, testicular lymphoma is very rare in pediatric and young adult patients. Differentiating between TARTs and non-germ cell tumors such as Leydig cell tumor or Sertoli cell tumor may also be a challenge. On ultrasound, these tend to demonstrate greater heterogenicity than TARTs and can sometimes be distinguished by areas of cystic necrosis, hemorrhage, or calcification.[10] Although sonographic differentiation of these testicular pathologies can be difficult, we have developed a review of the most salient sonographic features and propose a decision tree that relies on these specific features, with the goal of increasing clinician’s confidence in diagnosing TART.
Laterality
There has been no study to date with a sample size sufficient to determine the laterality of TART, but existing literature favors bilateral presentation. In Wang et al.’s retrospective study of 15 case reports, all 15 cases were bilateral. Delfino et al. reviewed sonographic studies in 11 cases where 9 were bilateral and 2 unilateral. Similarly, Stikkelbroek et al. found that 10 of 16 cases had bilateral sonographic findings.[11]
Echogenicity
Most TARTs are hypoechoic relative to the surrounding testicular parenchyma, with varying degrees of heterogeneity. Wang et al. found that 28 of 30 cases were hypoechoic. The remaining two cases were classified as having heterogeneous isoechogenicity. Of the cases that were hypoechoic, 17 of 30 demonstrated homogenous echogenicity and 11 of 30 heterogeneous echogenicity. Similarly, Delfino et al. found that 9 of 11 cases were hypoechoic and 2 of 11 cases were hyperechoic. No further classification of sonographic heterogeneity was performed.
Doppler flow
Prior studies have shown that TARTs demonstrate varying degrees of hypervascularity on Doppler imaging (Figure 3). Wang et al. found that 23 of 30 lesions demonstrated a rich blood supply and 7 of 30 a scarce blood supply. According to Delfino et al., 2 of 11 cases demonstrated perilesional flow and 1 of 11 case demonstrated both perilesional and intralesional flow. 8 of 11 cases were significant for no flow signal at all. These results indicate that vascularity can vary significantly between lesions. As such, we advise that it is important to rule out other pathologies, notably testicular lymphoma, when encountering sonographic evidence of hypervascularity. Testicular lymphoma presents similarly, with discrete hypoechoic intratesticular masses within a markedly hypervascular testis.[12]
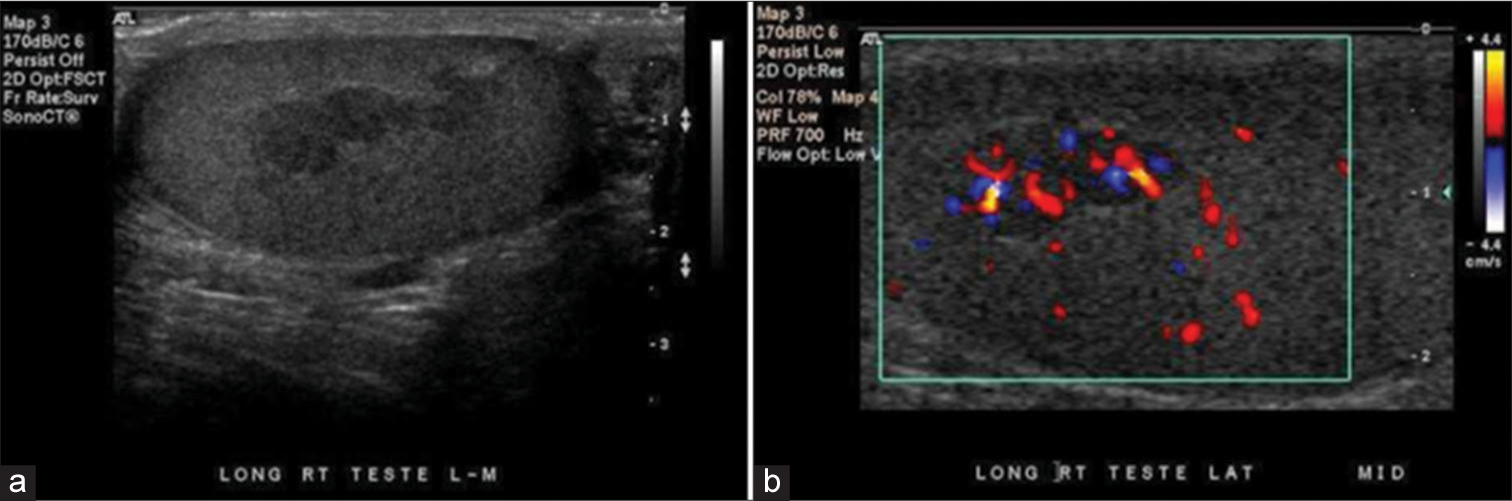
- 17-year-old patient with congenital adrenal hyperplasia. (a) Grayscale ultrasound demonstrates a lobulated, hypoechoic lesion along the length of the mediastinum testis, (b) color flow Doppler demonstrates significant vascularity within the lesion.
Location
The location of the vast majority of TARTs is adjacent to the mediastinum testis, although larger lesions may no longer be contained in this particular region. Following the stages of TARTs, the adrenal rest cells present in the rete testis and follow a pattern of hypertrophy and hyperplasia. All of Wang et al.’s 15 cases were documented to be near the mediastinum testis or at the testicular hilum. Similarly, Stikkelbroek et al. also confirmed the vast majority of cases to be adjacent to the mediastinum testis.
Border
The vast majority of previously documented TARTs showed well-defined boundaries but irregular shape. Wang et al. found that all 15 cases had clear boundaries, 25 of 30 lesions had an irregular shape, and 5 of 30 lesions had a round appearance. Stikkelbroek et al. also found that the majority of lesions are sharply marginated. Delfino et al. showed in specific cases that hypoechoic lesions tend to be more round, while hyperechoic lesions tend to be more elongated and tubular.
Calcification
Reviewing the existing literature and published cases of TARTs, investigators generally agree that there is no sonographic evidence of calcification. Wang et al. demonstrated in all 15 cases that there was no evidence of calcification. Calcification may be present in rare cases, as Stikkelbroek et al. briefly discussed the possibility of posterior calcifications in single heterogeneous lesion.
CONCLUSION
Although there are no clinical guidelines for testicular imaging of males with CAH, the presence of TARTs in this population is significant. Sonography is the first-line approach to assess the presence of TART, and although salient features have been studied, it is important to review these features and how these features may separate TARTs from other diagnoses, including lymphoma and non-germ cell tumors, notably Leydig cell tumors. Furthermore, TARTs are often an incidental finding and clinical radiologists should be aware of this benign mass in the setting of CAH to help prevent unnecessary surgical procedures. The decision tree we have proposed summarizes all of the most salient and agreed on sonographic findings with the goal of increasing clinical confidence when diagnosing TARTs (Figure 4).
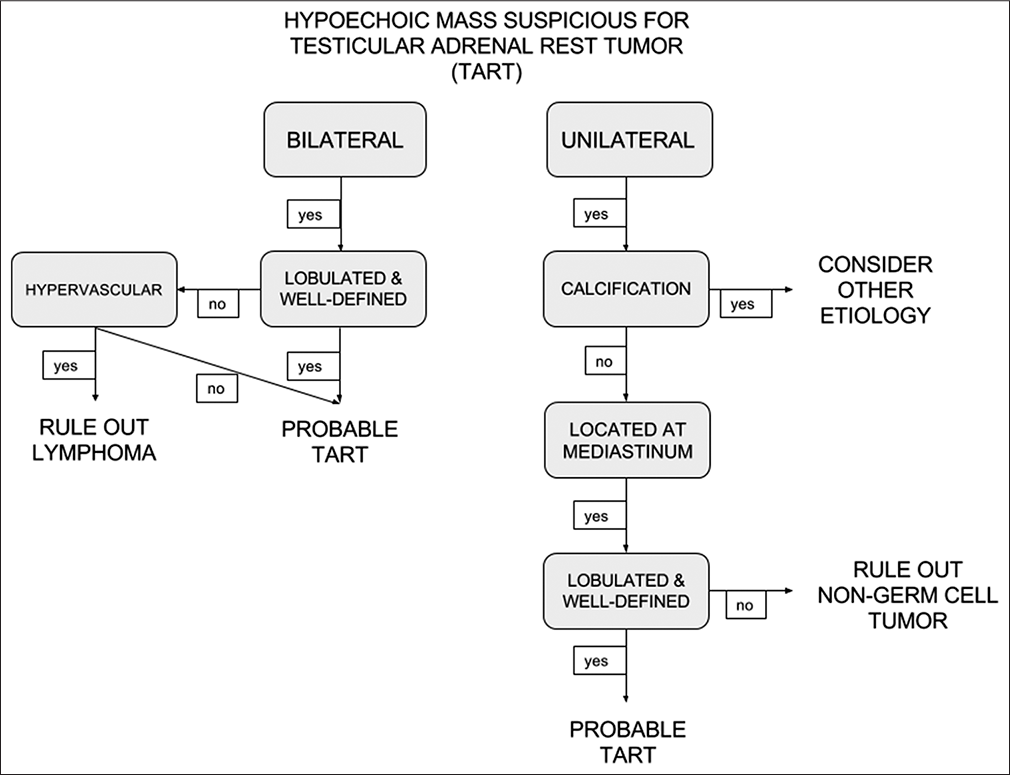
- Decision tree to aid in the diagnosis of testicular adrenal rest tumors.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vikram Dogra is on the Editorial Board of the Journal.
References
- Sonographic appearance of testicular adrenal rest tissue in congenital adrenal hyperplasia. J Ultrasound Med. 2004;23:979-81.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular adrenal rest tissue in congenital adrenal hyperplasia: Serial sonographic and clinical findings. AJR Am J Roentgenol. 1999;172:1235-8.
- [CrossRef] [Google Scholar]
- Diagnosis of testicular adrenal rest tumors on ultrasound. Medicine. 2015;94:e1471.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular adrenal rest tissue in congenital adrenal hyperplasia: Comparison of MR imaging and sonographic findings. AJR Am J Roentgenol. 1999;172:1003-6.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2009;2009:624823.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89:597-601.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia: Prevalence and sonographic, hormonal, and seminal characteristics. J Ultrasound Med. 2012;31:383-8.
- [CrossRef] [PubMed] [Google Scholar]
- Morphological approach to tumours of the testis and paratestis. J Clin Pathol. 2007;60:866-80.
- [CrossRef] [PubMed] [Google Scholar]
- Testicular adrenal rest “tumor” or leydig cell tumor? A report of a challenging case with literature review. Avicenna J Med. 2013;3:15.
- [CrossRef] [PubMed] [Google Scholar]
- High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86:5721-8.
- [CrossRef] [PubMed] [Google Scholar]







