Translate this page into:
Tacrolimus-induced Segmental Renal Artery Vasoconstriction in the Setting of Nicardipine Administration after Renal Transplantation

Corresponding Author: Matin Goldooz, Department of Radiology and Imaging Sciences, University of Utah Hospital, 30 North 1900 East, Salt Lake City, UT, 84132, United States. E-mail: matin.goldooz@utah.edu
-
Received: ,
Accepted: ,
How to cite this article: Goldooz M, Kennedy A, Campsen J. Tacrolimus-Induced Segmental Renal Artery Vasoconstriction in the Setting of Nicardipine Administration after Renal Transplantation. Am J Sonogr 2018, 1(12) 1-4.
Abstract
Immediate postoperative complications in renal transplants include renal artery thrombosis and dissection both of which carry significant risk for loss of the graft. We present an unusual case in which apparent devascularization of the upper pole of the transplant kidney was due to reversible vasospasm as a result of a drug interaction. Tacrolimus, a calcineurin inhibitor, is used for post-transplant immunosuppression. The antihypertensive medication nicardipine impairs liver metabolism of tactolimus and, in this case, the combination of drugs resulted in supratherapeutic levels of tacrolimus causing acute nephrotoxicity as well as profound vasoconstriction which was most pronounced in the upper pole branch renal artery and simulated devascularization of almost half of the transplant kidney. This case highlights the fact that not all abnormal post-transplant Doppler findings are due to surgical technique or embolic events and illustrates the importance of drug interactions in this group of patients with complex medical conditions.
Keywords
Nicardipine
Renal artery
Renal artery Doppler ultrasound
Renal transplantation
Tacrolimus
INTRODUCTION
Vascular complications after kidney transplantation are uncommon but remain an important clinical challenge. The most common is renal artery stenosis, which is a delayed complication happening 3–24 months after surgery.[1] Immediate post-transplant vascular complications are most often due to mechanical surgical complications. Transplant renal artery thrombosis occurs in 0.4–3.5% of recipients. It is most common in the immediate post-operative period due to mechanical problems such as vessel kinking or arterial dissection, but it may occur at any time following surgery. The typical clinical presentation is elevated serum creatinine with acute reduction in urine output.[2] Renal vein thrombosis is another serious vascular complication with a reported prevalence of 0.1–4.2%. It presents with pain, fever, and swelling in the area of the graft sometimes associated with edema of the ipsilateral lower extremity.[1,3]
Duplex Doppler sonography is crucial in the evaluation of transplant dysfunction as it allows non-invasive assessment of the transplant structure and vasculature without exposure to ionizing radiation or intravenous contrast material and, it can, if necessary, be performed at the patient’s bedside. The sonographic findings of vascular compromise are outlined in Table 1.[1]
| Timing | MRA | MRV | Arcuate arteries | |
|---|---|---|---|---|
| Renal artery stenosis | 3 months–2 years | PSV 2–300 cm/s | + | Tardus parvus, AT>0.08–0.1 s |
| Renal artery thrombosis | Most common in the immediate postoperative period | No flow | + | No flow |
| Renal vein thrombosis | Most common in the first postoperative week | High resistance with reversed end diastolic flow | No flow | Increased resistance |
RA: Main renal artery, MRV: Main renal vein, PSV: Peak systolic velocity, AT: Acceleration time
We present an unusual case in which an apparent segmental infarct of the graft was not due to arterial thrombosis but was caused by reversible, branch-renal-artery vasoconstriction secondary to tacrolimus toxicity in the setting of nicardipine therapy.
CASE REPORT
A 64-year-old male with hypertension and end-stage renal disease secondary to type I diabetes presented for deceased-donor renal transplantation. During surgery, the surgeon noted early bifurcation of the transplant renal artery with a smaller branch supplying the upper pole. The surgery was uneventful, and the patient was transferred to the surgical Intensive Care Unit per institutional protocol. Tacrolimus, mycophenolic acid, and methylprednisolone were started for immunosuppression and a nicardipine drip was started for blood pressure control. Before surgery, the patient had been taking calcium channel blockers (CCB) for hypertension.
On postoperative day #3, his urine output decreased and his serum creatinine increased. Ultrasound of the renal allograft showed appropriate arborization of vessels in the interpolar and lower pole areas but virtually undetectable flow in the upper pole even with power Doppler. The main renal artery showed rapid systolic upstroke but absent diastolic flow. Where detectable the waveform in the arcuate vessels of the upper pole showed a parvus tardus waveform (Figure 1). The immediate concern was for acute devascularization of the upper pole. On review of medication and laboratory test results, the baseline serum tacrolimus level had been significantly elevated to 41.2 ng/mL (normal 4–11 ng/mL), and further doses of tacrolimus had been held. By postoperative day #3, at the time of the decreased urine output, the level had dropped to18 ng/mL which was improved but still elevated.
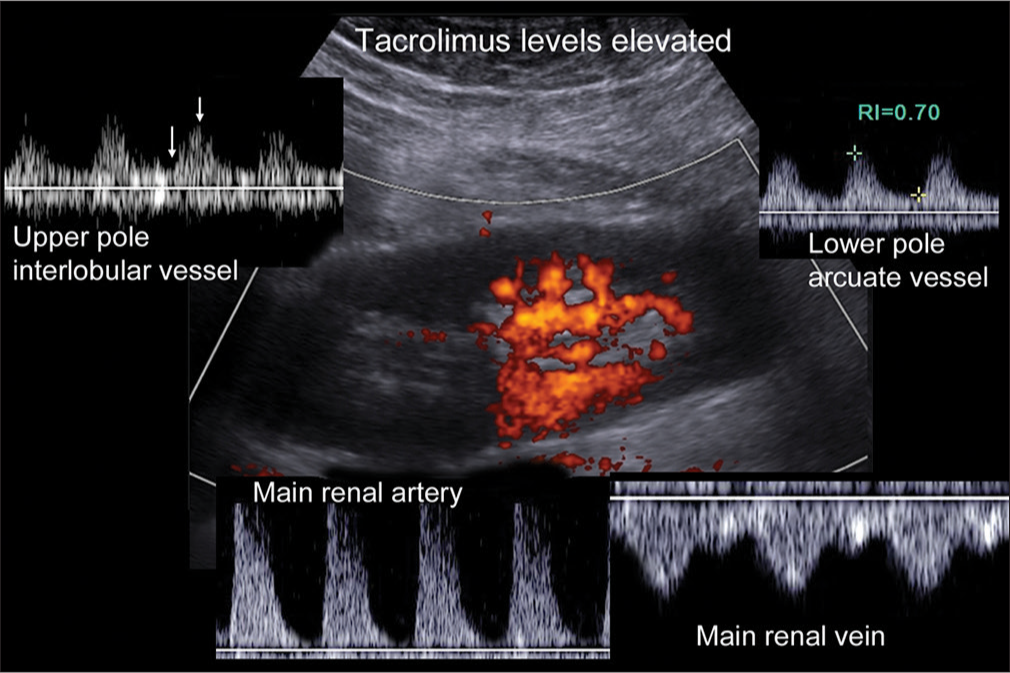
- Power and spectral Doppler in a 64-year-old male transplant recipient with diminished urine output and increasing serum creatinine. Power Doppler shows marked reduction in perfusion of the upper pole. Spectral Doppler shows abnormally high resistance in the main renal artery with absent end diastolic flow. The main renal vein was patent excluding renal vein thrombosis. The resistive index in the lower pole arcuate vessel was surprisingly normal, but acceleration time was subjectively increased. Spectral Doppler in the upper pole was challenging, but eventually, a signal was obtained from an interlobular vessel. The acceleration time is increased (arrows mark the onset of the systolic upstroke and the peak systolic velocity). Due to high tacrolimus levels at the time of the scan and the presence of an early branch to the upper pole from the main renal artery, he was placed on heparin on the assumption that the decreased flow was due to vasoconstriction rather than segmental infarction.
Tacrolimus is a potent vasoconstrictor. The anatomy of the renal allograft with a small branch artery to the upper pole, the lack of any operative manipulation of this branch, and the tardus parvus waveform in the territory of the smaller vessel all suggested that the reduction in flow was due to vasoconstriction which could be explained by tacrolimus toxicity. The timing was wrong for true renal artery stenosis which usually takes at least 3 months to develop and is most common at the site of the renal artery anastomosis. If there had been thrombosis of the branch renal artery, we should not have been able to detect any flow in the upper pole.[4] A published series of segmental renal infarction at one institution documented marginal graft use or significant intraoperative blood loss as the main causative factors; neither was applicable in our case. Primary graft non-function resulted in 62% of the case in that series; this was not seen in our patient. There was no correlation between infarct area and graft function, but they did note that long-term graft compromise was out of proportion to the extent of parenchymal loss.[5]
The decision was made not to return to the operating room but to wait for the tacrolimus levels to normalize. Anticoagulation was also started to minimize the risk of thrombosis. Repeat ultrasound 1 day later showed improved blood vessel arborization within the upper pole of the kidney (Figure 2) which would not have occurred in the setting of branch artery thrombosis or segmental renal artery stenosis. Two days later, the serum tacrolimus level had normalized to 6.9 ng/mL and urine output was normal and the serum creatinine returned to normal over the next few days supporting the working diagnosis that diminished function was due to global vasoconstriction with asymmetric involvement of the graft secondary to early bifurcation of the main renal artery with a relatively small vessel supplying the upper pole.
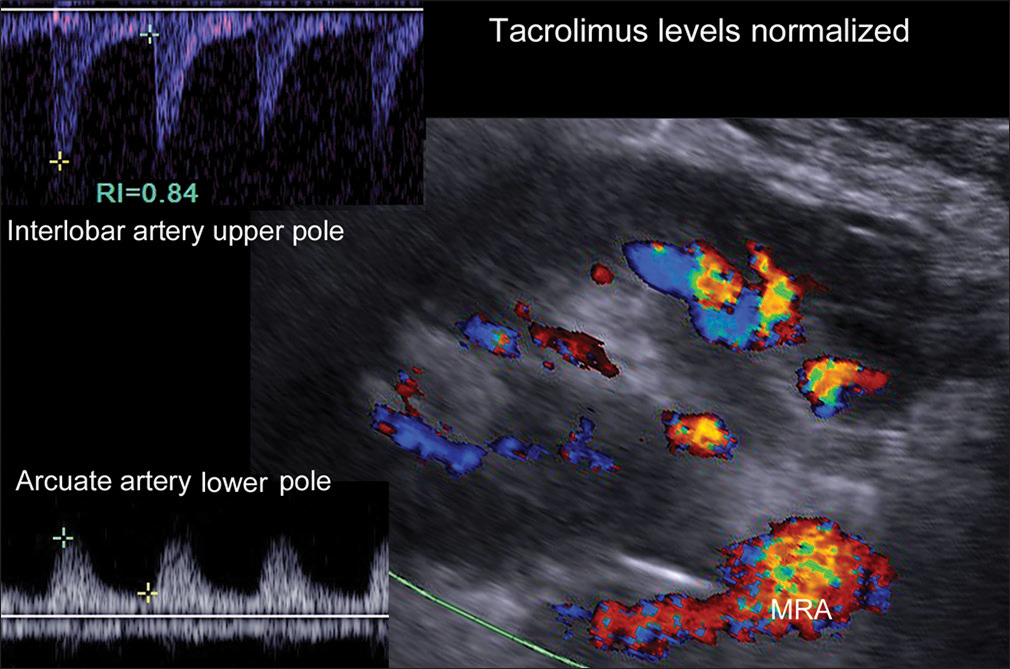
- Color and spectral Doppler one day later in the same patient shows definite flow to the upper pole detectable on color Doppler. At this point, his tacrolimus level was in the normal therapeutic range. The patient had developed a hematoma (attributed to heparinization), which altered the orientation of the kidney necessitating a different angle of insonation. The image has been rotated to allow direct comparison with Figure 1. Spectral Doppler showed stable flow to the lower pole and definite arterial flow to the upper pole. Over the next several days, the creatinine-normalized and the hematoma resolved. He was discharged from the hospital with normal renal function.
DISCUSSION
Tacrolimus is a calcineurin inhibitor (CNI) commonly used for immunosuppression following solid organ transplantation. The therapeutic benefits in the prevention of rejection are well established, but high tacrolimus levels are associated with acute nephrotoxicity.[6,7] Both acute reversible as well as insidious irreversible forms of CNI nephrotoxicity have been identified.[8] CNI-induced nephrotoxicity is likely due in part to arteriolar vasoconstriction, which has been shown to be mediated by overexpression of endothelin in animal models.[9] More systemic vasoconstriction with CNI therapy has been observed in humans, including in the setting of the acute coronary syndrome.[10,11] Reversible histological changes have also been demonstrated including, on rare occasions, thrombotic microangiopathy.[12-14]
CCBs such as nicardipine are commonly used to manage hypertension both before and after renal transplantation. Nicardipine inhibits the cytochrome P450 enzyme CYP3A4, which along with CYP3A5 is involved in the metabolic pathway for tacrolimus in the liver.[15] Use of nicardipine together with tacrolimus may increase the risk of supratherapeutic levels of tacrolimus, particularly in Caucasian patients who more commonly do not express CYP3A5.[15]
While tacrolimus toxicity does not cause post-transplant renal failure as commonly as mechanical vascular lesions, it is still an important consideration particularly when drug interactions may increase the risk of elevated serum tacrolimus levels. Doppler ultrasound is the initial imaging modality of choice whenever there is a clinical concern for vascular compromise as it can identify potentially-treatable, hemodynamically-significant vascular lesions.[16]
CONCLUSION
Not all abnormal post-transplant Doppler findings are due to surgical technique or embolic events. This case highlights the potential for serious complications as a result of a drug toxicity and interactions between CCBs and CNIs. Such drug combinations are to be expected in the population requiring renal transplantation as patients with end-stage renal disease are frequently hypertensive and persistent hypertension postoperatively can shorten graft survival.[17]
A careful review of the drug history is as important as ultrasound evaluation of the graft. In this case, the patient was spared an unnecessary return to the operating room with a final diagnosis of transient tacrolimus toxicity with severe branch-renal-artery vasoconstriction.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Imaging complications of renal transplantation. Radiol Clin North Am. 2016;54:235-49.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular complications in renal transplantation: A single-center experience in 1367 renal transplantations and review of the literature. Transplant Proc. 2009;41:1609-14.
- [CrossRef] [PubMed] [Google Scholar]
- Complications of renal transplantation. Radiographics. 2005;25:1335-56.
- [CrossRef] [PubMed] [Google Scholar]
- Segmental infarction with graft dysfunction: An emerging syndrome in renal transplantation? Nephrol Dial Transplant. 2002;17:123-8.
- [CrossRef] [PubMed] [Google Scholar]
- Tacrolimus-induced nephrotoxicity and genetic variability: A review. Ann Transplant. 2012;17:111-21.
- [CrossRef] [PubMed] [Google Scholar]
- The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS One. 2014;9:e111128.
- [CrossRef] [PubMed] [Google Scholar]
- Biomarkers of calcineurin inhibitor nephrotoxicity in transplantation. Biomark Med. 2014;8:1247-62.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. 1993;91:2144-9.
- [CrossRef] [PubMed] [Google Scholar]
- Immunosuppressive therapy induced coronary vasospasm and acute myocardial infarction in a patient undergoing new renal transplantation. Postepy Kardiol Interwencyjnej. 2015;11:141-5.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic and renal hemodynamic differences between FK506 and cyclosporine in liver transplant recipients. Transplantation. 1993;55:1332-9.
- [CrossRef] [PubMed] [Google Scholar]
- Reverse diastolic intrarenal flow due to calcineurin inhibitor (CNI) toxicity. Am J Transplant. 2006;6:1963-7.
- [CrossRef] [PubMed] [Google Scholar]
- Calcineurin inhibitor toxicity in a renal transplant recipient. NDT Plus. 2009;2:175-6.
- [CrossRef] [PubMed] [Google Scholar]
- Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol. 2006;2:398-404.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of tacrolimus toxicity in CYP3A5 nonexpressors treated with intravenous nicardipine after kidney transplantation. Transplantation. 2012;93:806-12.
- [CrossRef] [PubMed] [Google Scholar]
- Duplex assessment of the renal transplant. Surg Clin North Am. 1990;70:133-41.
- [CrossRef] [Google Scholar]
- Assessment and management of hypertension in transplant patients. J Am Soc Nephrol. 2015;26:1248-60.
- [CrossRef] [PubMed] [Google Scholar]







