Translate this page into:
Acoustic Streaming: A Diagnostic Clue to Diagnosing Testicular Teratoma

Corresponding Author: Alexander Croake, Department of Imaging Sciences, University of Rochester Medical Center, 601 Elmwood Avenue, Rochester, New York 14642. E-mail: alexander_croake@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Croake A, Croake MF, Dogra V. Acoustic Streaming: A Diagnostic Clue To Diagnosing Testicular Teratoma. Am J Sonogr 2019, 2(3) 1-4.
Abstract
Teratomatous tissue is commonly seen in a variety of malignant testicular tumors, and while the exact determination of testicular tumor subtypes heavily relies on pathologic diagnosis, ultrasound remains the gold standard in the initial evaluation of such entities. The major groups of testicular tumors may demonstrate characteristic features which can point the radiologist toward a more pruned differential diagnosis. While it is important for the interpreting physician to be aware of such features, it is of equal necessity that they are aware of potential visual phenomena, such as acoustic streaming in the diagnosis of testicular tumors. We present a case of a testicular teratoma with acoustic streaming. This testicular tumor was confirmed to be predominantly a teratoma with a minimal element of seminoma on histopathology.
Keywords
Testicular teratoma
Mixed germ cell tumor
Non-seminomatous germ cell tumor
Ultrasound
Acoustic streaming
INTRODUCTION
Intratesticular malignancies account for approximately 1% of malignancies experienced by men.[1] Germ cell tumors of the testicle are the most common, the majority of which are seminomas. The other major categories of germ cell tumors of the testicle are non-seminomatous germ cell tumors, which is a term that encompasses mixed germ cell tumors, teratomas, yolk sac tumors, embryonal cell tumors, and choriocarcinomas. Of these, mixed germ cell tumors are the most common, typically containing components of teratomas and embryonal cell carcinoma. Non-seminomatous germ cell tumors are characterized by their sonographic heterogeneity, often containing both solid and non-solid components. Lesions which contain a larger cystic or fluid component can be prone to visual phenomenon, such as acoustic streaming. Here, we demonstrate the sonographic phenomenon of acoustic streaming in a patient with a predominantly teratomatous mixed germ cell tumor of the testicle, a finding that has not previously been published.
CASE REPORT
A 27-year-old man with a medical history of paranoid schizophrenia, morbid obesity, pilonidal cyst, diabetes mellitus, hypertension, hyperlipidemia, hypogonadism, and phimosis presented to his primary care physician with the chief complaint of the right scrotal swelling, scrotal erythema, and mild pain for the prior 3 days. The patient had already established care with urology on an outpatient basis 8 years prior for vague scrotal symptoms at the age of 19, although intermittent follow-up over the years had proved unrevealing. The patient was now sent to the emergency department for further evaluation of suspected testicular torsion.
At present, the patient denied antecedent trauma or injury and complained of no associated genitourinary or constitutional symptoms. Physical examination revealed a grossly indurated right hemiscrotum. No erythema or fluctuance was noted. There was mild tenderness during this examination.
Scrotal ultrasound was performed, revealing a tensely expansile, heterogeneously complex mass lesion within the right scrotum, which both replaced and substantially peripheralized the normal residual testicular parenchyma (Figure 1). This lesion was comprised a large component of complex fluid with little heterogeneous solid tissue. Acoustic streaming was observed in this testicular mass (Figure 2, Video 1). The ipsilateral epididymis was heterogeneously enlarged, with multiple foci of hyperechogenicity, but without posterior acoustic shadowing, likely related to chronic epididymitis. The intratesticular mass did not demonstrate hyperemia. The overlying scrotal skin was diffusely thickened and edematomous. There was just a fine rim of normal testicular parenchyma pushed to the periphery. The left scrotum and its contents were sonographically normal.
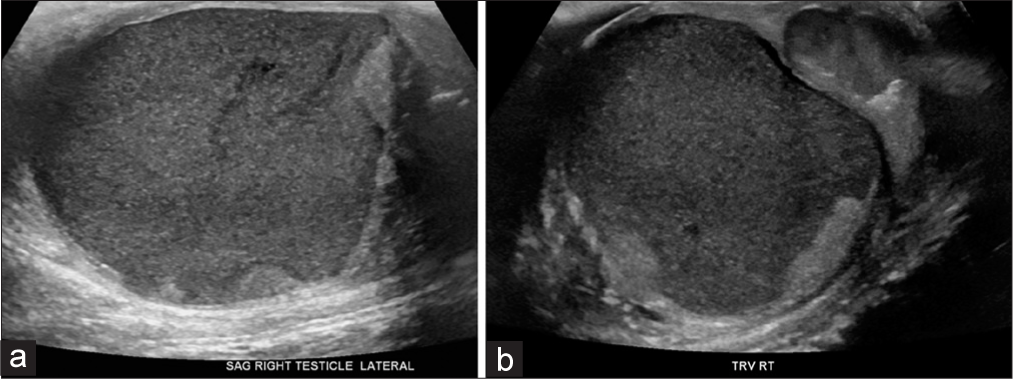
- A 27-year-old man presented to the emergency department with several days of the right scrotal swelling, erythema, and mild pain. Sagittal (a) and transverse (b) sonographic images through the right testicle demonstrate gross replacement of the normal testicular parenchyma with a complex fluid collection, with peripheralization of the scant residual testicular parenchyma.
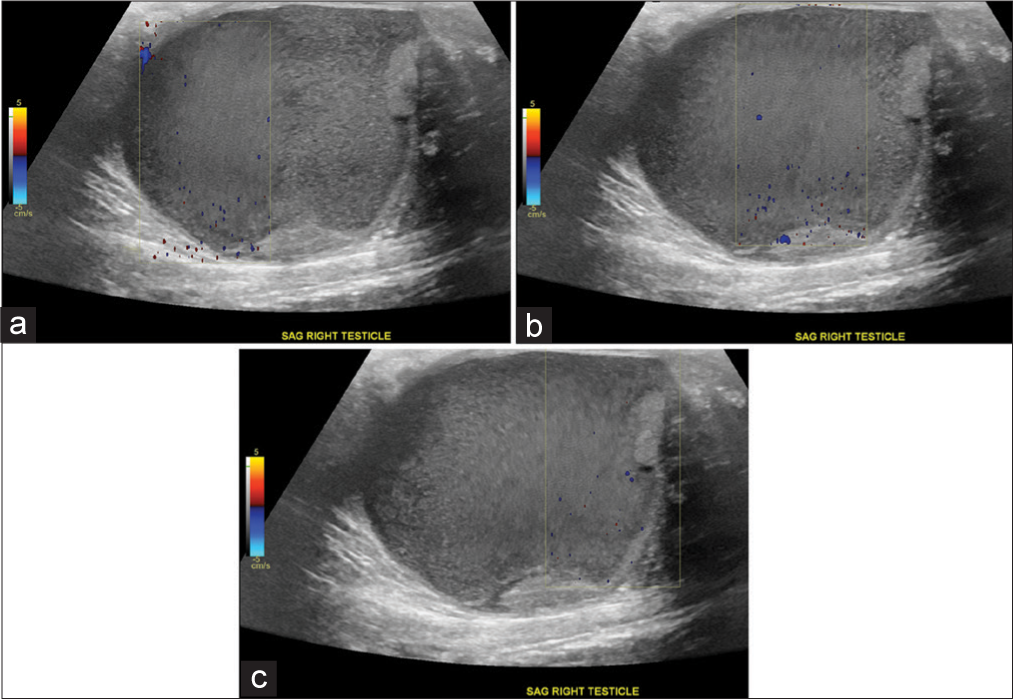
- A 27-year-old man who presented to the emergency department with several days of the right scrotal swelling, scrotal erythema, and mild pain. Doppler ultrasound images of large complex fluid collection replacing the right testicle in the sagittal plane (a, b, c) demonstrate perception of fluid movement through acoustic streaming even on serial still captures, focused within Doppler tracer box.
Initial workup demonstrated tachycardia and a leukocytosis to 19,000/uL. These findings, in conjuncture with the significant lesional fluid component and scrotal hyperemia, lead to the presumptive diagnosis of epididymo-orchitis, with possible associated pyocele and testicular abscess. Underlying malignancy was suggested by the attending radiologist as a differential diagnosis. The patient was started on intravenous vancomycin and Zosyn empirically, and the patient was admitted by urology for further management. Tumor markers, including beta-HCG and alpha-fetoprotein, were within normal limits, and testosterone was depressed, at 80 ng/dL. Given the persistent concern for underlying malignancy, the patient was taken to the operating room for radical orchiectomy after medical optimization.
Orchiectomy was uneventful, and at the time of surgery, no significant invasion of the tunica albuginea was appreciated. Grossly, the specimen showed replacement of the normal testicle by a multilobulated cystic structure which contained gelatinous dark green/brown sludge, with a periphery of fibrotic tissue (Figure 3). Histopathology demonstrated gross replacement of normal testicular parenchyma by regions of cystic change with associated hemorrhage interspersed with swaths of cartilage. Nests of seminomatous components were also identified, with characteristic prominent nucleoli and clear cytoplasm. A small rim of normal testicular parenchyma was identified (Figure 4). The final diagnosis of mixed germ cell tumor consisting of 90% teratoma and 10% seminoma was rendered.
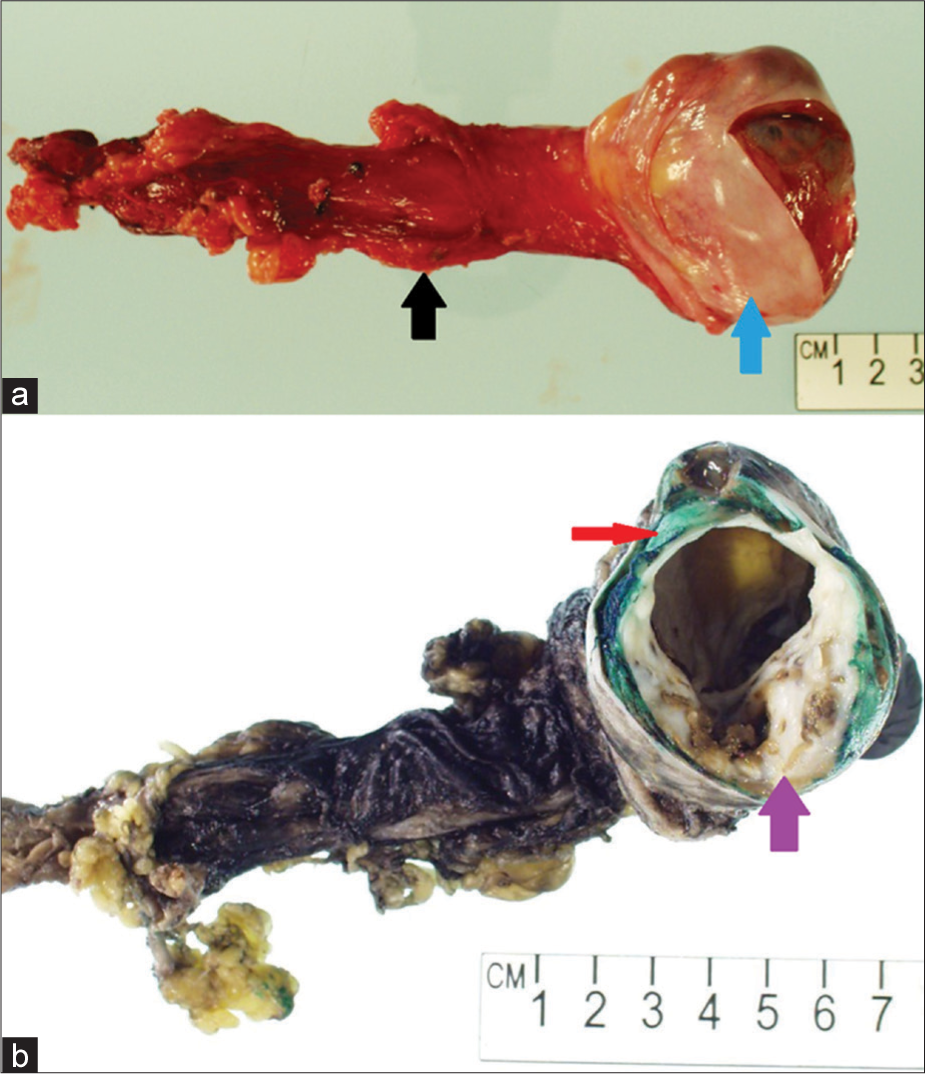
- A 27-year-old man who presented to the emergency department with several days of the right scrotal swelling, scrotal erythema, and mild pain. Gross photographs taken of the surgical specimen (a) and after inking in the pathology laboratory (b) demonstrate resection specimen including the spermatic cord (black arrow) and testicle (blue arrow). There is gross heterogeneous replacement of the normal testicular parenchyma by a multicystic neoplasm with necrosis (purple arrow) and a small peripheral rind of normal testicular parenchyma (red arrow).
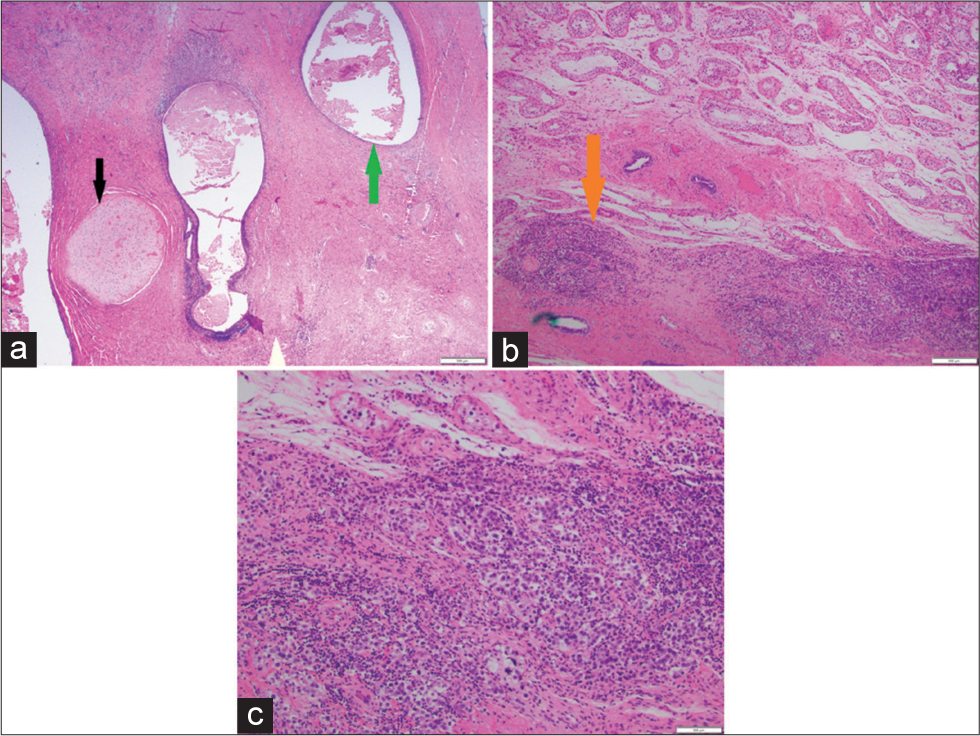
- A 27-year-old man who presented to the emergency department with several days of the right scrotal swelling, scrotal erythema, and mild pain. Histopathologic sections through the testicle at low power (a) demonstrate nests of pink cartilaginous deposits (black arrow) interspersed with vacuoles hemorrhage (green arrow). Successively higher power microscopic images (b, c) demonstrate focal areas of higher cellularity, the individual cells of which are characterized by prominent nucleoli and clear cytoplasm, suggesting a seminomatous component (orange arrow). Final diagnosis of mixed germ cell tumor consisting of 90% teratoma and 10% seminoma.
DISCUSSION
The two major designations of testicular malignancies are primary germ cell tumors and non-germ cell tumors. The vast majority of testicular tumors are primary germ cell tumors. Within this, we can further subclassify tumors into seminomas and non-seminomatous germ cell tumors (NSGCT). Of these, seminomas are the most common, accounting for upward of 45% of all primary testicular tumors. Regarding NSGCT, mixed germ cell tumors are the most common.[2] While there is a degree of overlap between the presentation and diagnosis of these entities, there are certain imaging features which can aid in populating a more accurate differential diagnosis.
Seminomas, as with most testicular neoplasms, present classically as a painless and palpable unilateral testicular mass. Depending on the stage of disease at the time of presentation, as well as patient comorbidities, other symptoms, such as back or abdominal pain, may be present. The first-line imaging modality in evaluation of a testicular mass is ultrasound. Typical seminomas will manifest as a homogeneous intratesticular mass, which is often hypoechoic when compared to the normal testicular parenchyma. They are usually well defined, and lacking in local invasion, as delineated by the tunica albuginea. Intralesional vascularity will be present on Doppler imaging.
NSGCT is a term that encompasses a variety of subtypes of testicular malignancies (including mixed germ cell tumors, teratomas, yolk sac tumors, embryonal cell tumors, and choriocarcinomas), and while these vary primarily based on histology, there are general imaging characteristics that help differentiate this group from seminomas. The clinical presentation is similar to that of seminomas, although NSGCT tends to occur in younger patients, most commonly in the second and third decades of life. On ultrasound, NSGCT tends to be more heterogeneous, with both solid and cystic components, and often with foci of calcifications. Involvement and invasion of the tunica albuginea is seen more commonly, and thus, these tumors tend to be more clinically aggressive.
Teratomatous tissue is seen as a component of the majority of mixed germ cell tumors; however, pure testicular teratomas can also occur, representing upward of 10% of all testicular tumors.[3] We can further subdivide these tumors into two categories, largely delineated by their degree of microscopic differentiation: Mature and immature teratomas. Mature testicular teratomas demonstrate more well-differentiated tissue components, classically including those tissues derived from the embryologic endoderm, ectoderm, and mesoderm precursor cells. Thus, all of these tissue types may be present, which in part accounts for the classic heterogeneity of these tumors seen on ultrasound. At imaging, mature testicular teratomas show a generous cystic component with complex and heterogeneous internal echogenicity, with solid components of equally as variable echogenicity.
One of the great advantages of ultrasound, across all of medical imaging, is its ability to visualize a process in real time, in its living state. Perception of movement on ultrasound is not uncommon. Real-time movement on ultrasound forms the basis of vascular studies through Doppler imaging, echocardiograms, and provides exciting clips for expectant parents on prenatal scans. On gray scale imaging, too, visualization of particulate motion in fluid or complex cystic structures is common. This is due to interactions between the ultrasonographic sound waves and the fluid through which it is traveling. The postulation of steady flow within a liquid secondary to the associated propagation of sound within that fluid was first recognized by Michael Faraday, in 1831. Further investigations into this observation have revealed two predominating physical theories as to why this occurs. The first suggests that sound waves directly and independently interact with the fluid medium itself, causing motion. The second relies on sound wave interactions with not only the fluid medium itself but also in combination with interactions of the solid boundaries surrounding the medium.[4] The latter is what is now known as “acoustic streaming,” which we currently define as a non-linear steady flow of fluid driven by the absorption and propagation of high amplitude acoustic emissions.[5] More simply, we can observe movement which occurs due to interactions between sound waves, a fluid, and its solid boundaries.
Numerous reports of acoustic streaming in medical imaging have been published, including that occurring in cysts, ventricular fluid, and blood, across anatomy ranging from breasts to ovaries to the central nervous system.[6-8] Additional studies have attempted to determine the uniformity of acoustic streaming in certain pathologies, as well as its diagnostic value. For example, Edwards et al. assessed for acoustic streaming in a variety of cystic adnexal lesions and found that while other lesions did demonstrate this phenomenon; adnexal endometriomas invariably did not, suggesting that this may be a diagnostic adjunct.[9] Further investigations into acoustic streaming velocity ranges are ongoing. Similarly, in cases such as the one described here, acoustic streaming manifests as finite visualization of debris particles within the complex and viscous necrotic/liquefactive/tumefactive fluid within the right hemiscrotum, put into steady motion by incident ultrasonographic waves. At present, acoustic streaming has not been described in relation to neoplastic testicular pathology in literature, although the “filarial dance” characteristic of parasitic infections of the testes has been observed and reported.[10]
Teratomas have large cystic components, and the fluid they contained is similar to the composition of cerebrospinal fluid; it contains copious mucoid material and fat. Such fluids within lesions that demonstrate a cystic component may have fluid-fluid or fluid-fat levels. Due to these features, these tumors are likely to demonstrate acoustic streaming. Assessment for acoustic streaming may, therefore, prove to be an additional useful tool when evaluating mixed germ cell tumors with dominant teratomatous element versus those that are pure germ cell tumors. While the presence of acoustic streaming is not a specific finding for testicular teratomas, the familiarity and commonality of this phenomenon among radiologists may lead to the presumption of benignity. Thus, it is imperative that if acoustic streaming is visualized within a complex, heterogeneous collection, the radiologist maintains a degree of suspicion for potentially unexpected pathology, including malignancy.
CONCLUSION
Non-seminomatous germ cell tumors, particularly the mixed variety, generate a spectrum of sonographic findings at the time of initial diagnosis. Variability in sonographic findings in mixed germ cell tumors is largely a reflection of their vastly different histologic components. This complex variability can lead to the presence of visual phenomenon, such as acoustic streaming, which should not deter the radiologist from suggesting neoplastic processes like teratomas when populating a differetial diagnosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vikram Dogra is on the Editorial Board of the Journal.
References
- Tumors of the testis, adnexa, spermatic cord, and scrotum. In: Atlas of Tumor Pathology, Fascicle 25, Series 3. Washington, DC: Armed Forces Institute of Pathology; 1999. p. :1-290.
- [Google Scholar]
- Epidermoid cyst and teratoma of the testis: Sonographic and histologic similarities. J Ultrasound Med. 2005;24:1403-9.
- [CrossRef] [PubMed] [Google Scholar]
- Acoustic streaming in liquids. Proc R Soc Ser A Math Phys Sci. 1954;226:43-50.
- [CrossRef] [Google Scholar]
- Acoustic streaming generated by a focused gaussian beam and finite amplitude tonebursts. Ultrasound Med Biol. 1993;19:167-76.
- [CrossRef] [Google Scholar]
- Measurement of acoustic streaming using magnetic resonance. Ultrasound Med Biol. 2000;26:321-33.
- [CrossRef] [Google Scholar]
- A novel ultrasonic technique for differentiating cysts from solid lesions: Preliminary results in the breast. Ultrasound Med Biol. 1995;21:745-51.
- [CrossRef] [Google Scholar]
- Color doppler detection of acoustic streaming in a hematoma model. Ultrasound Med Biol. 2001;27:1255-64.
- [CrossRef] [Google Scholar]
- Acoustic streaming: A new technique for assessing adnexal cysts. Ultrasound Obstet Gynecol. 2003;22:74-8.
- [CrossRef] [PubMed] [Google Scholar]
- Live adult worms detected by ultrasonography in human bancroftian filariasis. Am J Trop Med Hyg. 1994;50:753-7.
- [CrossRef] [PubMed] [Google Scholar]







