Translate this page into:
Sonographic Diagnosis of Unilateral Synchronous Testicular Tumors

Corresponding Author: Allison Forrest, Department of Imaging Sciences, University of Rochester Medical Center, 601 Elmwood Ave, Box 214, Rochester, New York 14642, United States. E-mail: allison_forrest@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Forrest A, Numbere N, Jean-Gilles J, Frye T, Dogra V. Sonographic Diagnosis of Unilateral Synchronous Testicular Tumors. Am J Sonogr 2019; 2(2) 1-4.
Abstract
Testicular cancer accounts for 1% of all male cancers yet is the most common cancer affecting men aged 15–44 years. Most testicular cancers are seminomas or non-seminomatous germ cell tumors. Rarely, multiple testicular cancers may occur simultaneously, most often of the same histological type. However, synchronous tumors of different histological types may occur, although rarely. In this case study, we present the sonographic features with histopathologic correlation in a case of unilateral synchronous testicular tumors of discordant histology.
Keywords
Ultrasound
Testicular cancer
Synchronous testicular tumors
Mixed germ cell tumor
Seminoma
INTRODUCTION
Testicular cancer is uncommon, accounting for 1% of all male cancers, yet is the most common cancer affecting men aged 15–44 years.[1] Testicular germ cell tumors (TGCTs) represent 95% of testicular cancers and encompass three histological types: Seminomas, non-seminomas, and spermatocytic tumors.[2,3] Seminomas account for 30–50% of TGCTs and are diagnosed at a median age between 35 and 39 years.[1,2] Non-seminomas account for 44% of TGCTs and include embryonal carcinoma, yolk sac tumor, choriocarcinoma, teratoma, and mixed germ cell tumors. Non-seminomas are diagnosed at a median age between 25 and 29 years.[1]
Testicular tumors may be synchronous or metachronous, with both these diagnoses representing 1–5% of all cases of testicular cancer.[1,4-6] Although synchronous tumors with discordant pathologies have been reported, the majority of synchronous tumors have been identified in patients with bilateral tumors with concordant pathologies.
In this case study, we present the sonographic features with histopathologic correlation in a case of unilateral synchronous testicular tumors of discordant histology, a rare finding.
HISTORY
A 40-year-old male presented to his primary care physician with 10 months of right testis enlargement and firmness associated with aching pain. Physical examination showed a slightly enlarged, non-tender, and firm right testicle. The left testicle was normal on palpation. His primary care physician ordered an ultrasound examination and referred him to a urologist. Laboratory studies revealed an elevated alpha-fetoprotein (AFP) of 16 IU/mL (normal <10 IU/mL), a normal lactate dehydrogenase (LDH) of 205 U/L, and a normal beta-human chorionic gonadotropin (ß-HCG) of <1 mIU/mL.
Imaging Findings
Ultrasonography (US) of the scrotum demonstrated two solid right intratesticular lesions (Figure 1). The first lesion was a 3.3 × 2 × 1.8 cm hypoechoic solid mass. The second lesion was a heterogeneous, slightly hypoechoic lesion that demonstrated cystic changes and measured 2.6 × 2.1 × 2.4 cm. Color Doppler showed internal flow in both lesions, greater in lesion one than in lesion two. The right testis measured 2.6 × 3.9 × 4.3 cm. There was no intratesticular mass in the left testis. Chest X-ray showed no evidence of disease. No other pre-operative imaging workup occurred.
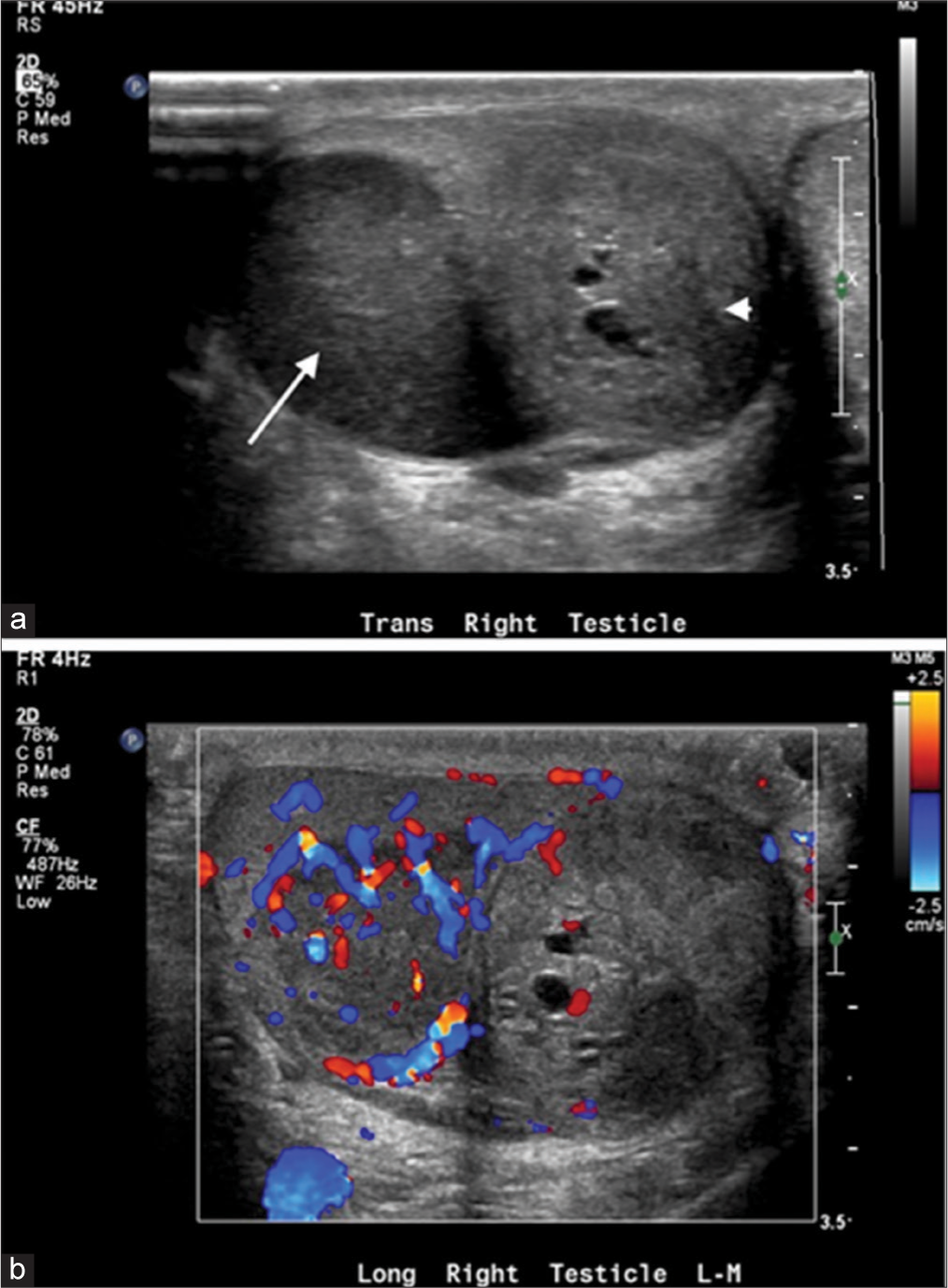
- A 40-year-old male with synchronous testicular tumors. (a) Gray scale US of the right testis demonstrates two intratesticular masses. There is a solid hypoechoic mass that measures 3.3 cm (arrow) and a heterogeneous, slightly hypoechoic lesion with cystic changes (arrowhead) that measure 2.6 cm. (b) Color Doppler demonstrates increased vascularity in the solid mass. The mass with cystic changes is relatively hypovascular.
Pathologic Examination
The patient underwent a right radical orchiectomy. Gross pathology showed two distinct masses (Figure 2). The first mass, which corresponded to the hypoechoic solid lesion on ultrasound, was pale tan-white, rubbery, and lobular. The mass was confined within the tunica albuginea without involvement of the spermatic cord or epididymis. Histology reported a seminoma, 4.2 cm in greatest dimension. Immunostains were positive for CD117 and negative for CD30 and glypican-3, confirming the diagnosis (Figure 3). The second lesion, which corresponded to the heterogeneous, slightly hypoechoic cystic lesion, was yellow-tan to gray-pink and also confined to the tunica albuginea without involvement of the spermatic cord or epididymis. Histologically, it was a 2.3 cm mixed germ cell tumor with extensive tumor necrosis, comprised 60% embryonal carcinoma, 30% teratoma, and 10% yolk sac tumor. Immunostains were positive for CD30 in the embryonal carcinoma component and positive for glypican-3 in the yolk sac tumor component, confirming the diagnosis (Figure 3). The patient was diagnosed with clinical Stage I (pT2NxM0) disease.
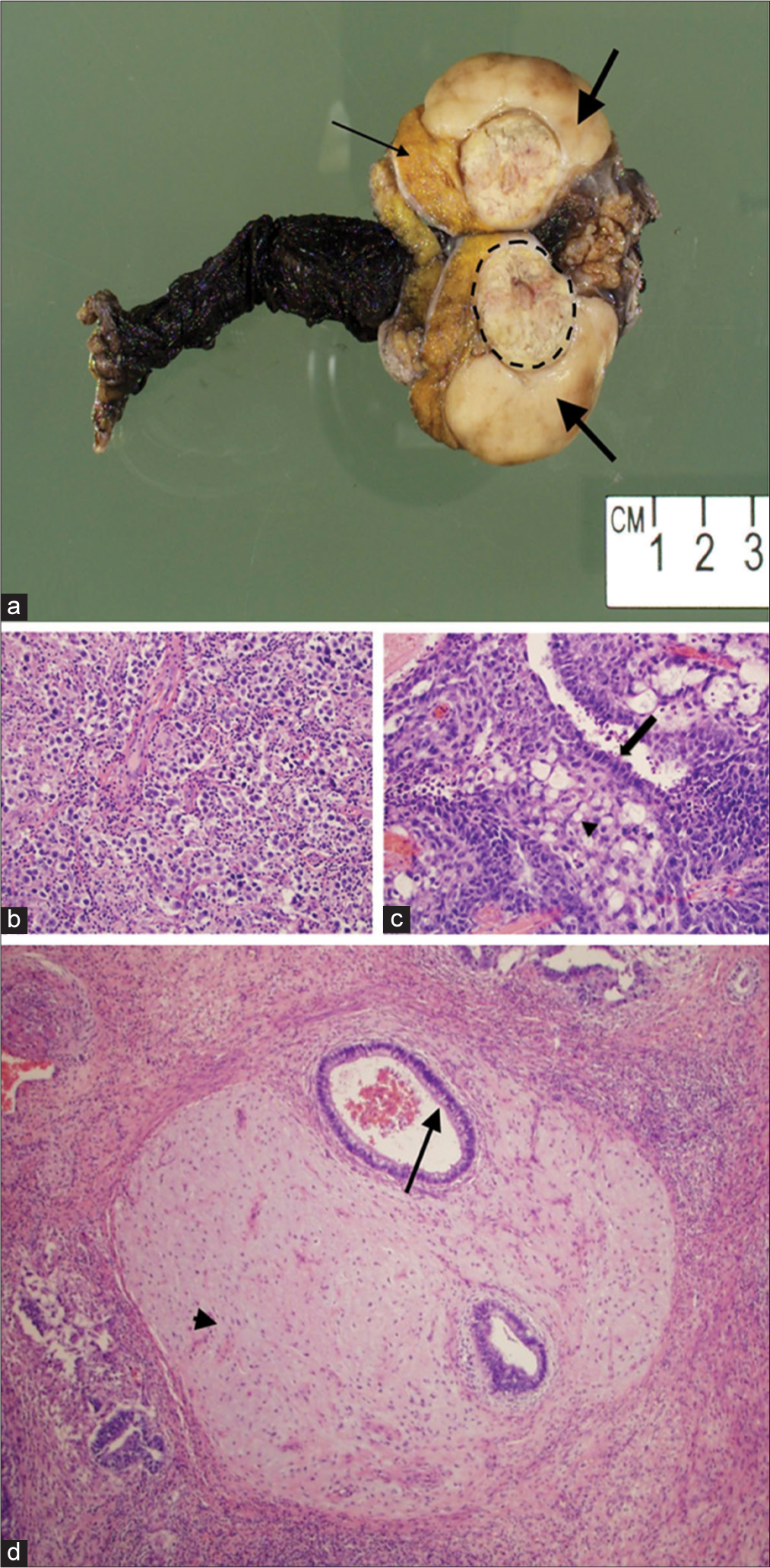
- A 40-year-old male with synchronous testicular tumors. (a) Photograph of the excised testis demonstrates a 4.2 cm seminoma (thick arrows), 2.3 cm mixed germ cell tumor (dashed circle), and normal testicular parenchyma (thin arrow). (b) High-power (20x) hematoxylin and eosin stained (H and E) image of the seminoma. (c) High-power (20x) H and E image of the embryonal carcinoma (arrow) and yolk sac tumor (arrowhead) component of the mixed germ cell tumor. (d) High-power (4x) H and E image demonstrates the teratoma portion of the mixed germ cell tumor containing cartilage (arrowhead) and bronchial glandular epithelium (arrow).
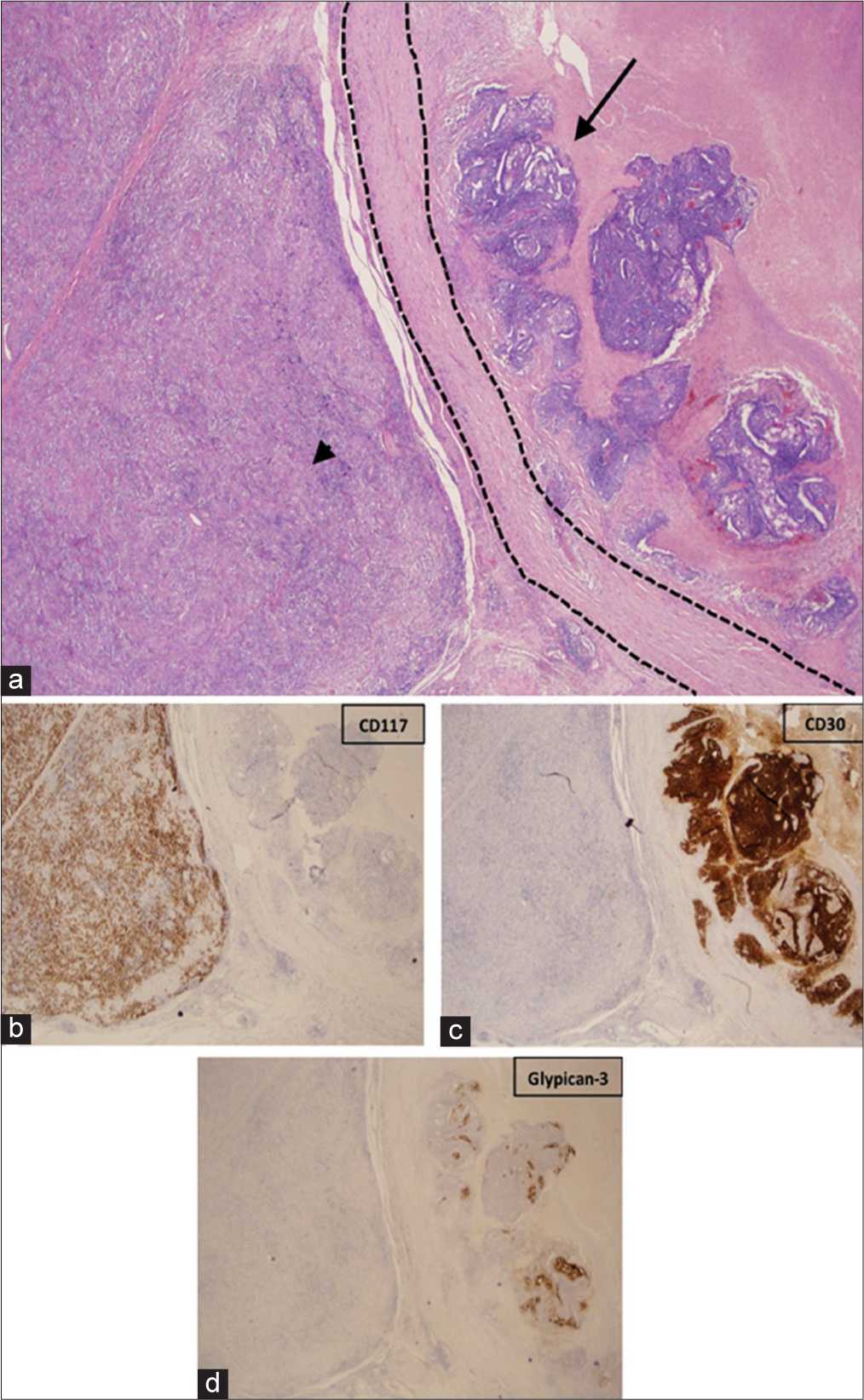
- A 40-year-old male with synchronous testicular tumors. (a) Low-power (2x) hematoxylin and eosin (H and E) stained image demonstrates seminoma (arrowhead) and mixed germ cell tumor (arrow) with a fibrous capsule separating the two tumors (dashed lines). (b) CD117 immunohistochemical stain highlighting the seminoma tumor. (c) CD30 immunohistochemical stain highlighting the embryonal carcinoma. (d) Glypican-3 immunohistochemical stain highlighting the yolk sac tumor.
Patient Follow-up
Post-operative tumor markers showed normal levels of AFP, HCG, and LDH. Computed tomography (CT) of the chest, abdomen, and pelvis showed no evidence of metastatic disease. Although adjuvant chemotherapy was offered, the patient elected to pursue disease surveillance. At 3-month and 6-month follow-up, surveillance with CT of the abdomen and serum tumor markers showed no sign of disease recurrence.
DISCUSSION
The presence of unilateral synchronous testicular tumors of discordant histology is a rare finding.[7] Most publications on synchronous testicular tumors have described bilateral tumors with a wide range of reported rates of cases with discordant histology. In an analysis of 3984 patients with testicular cancer, Holzbeirlein et al. identified 58 patients (1.5%) with bilateral tumors, 10 of which were synchronous (17%) and 7 (70%) of which had discordant pathology.[8] de Cassio Zequi conducted a review of bilateral TGCTs and found that 32.2% of the 261 synchronous bilateral tumors had discordant histology.[9] Discordant histology is similarly rare in unilateral tumors, as described by Cicero et al. In a retrospective study of 492 testicular tumors, Cicero et al. identified 9 patients (1.83%) with multiple, synchronous lesions of different histology within the same testis.[7] The largest seminoma non-seminoma pair reported in Cicero et al. was 2.0 cm and 3.5 cm, respectively. In this case study, we have reported the largest unilateral synchronous seminoma and non-seminoma pair, at 4.2 cm and 2.3 cm, respectively.
Cicero et al. described that most of the identified discordant tumors had distinct ultrasonographic appearances, an important consideration for radiologists, as identification of tumors with possible discordant histology can guide pathological examination and treatment options.[7] Lesions with different histology should be suspected when the masses have different echotexture, as seen in the presented case report.
Ultrasound appearance reflects gross morphology, which differs among the different types of testicular tumors. Seminomas are typically uniformly hypoechoic on US and may be lobulated or multinodular.[2] Non-seminomatous germ cell tumors are more variable in their ultrasound characteristics. These tumors are more likely to be heterogeneous with areas of increased echogenicity due to calcifications and hypoechoic areas due to the presence of cysts.[2] Mixed germ cell tumors are more common than pure forms of germ cell tumors, further increasing the heterogeneity of the ultrasonographic appearance of these tumors.[2]
In patients who present with testicular masses, magnetic resonance imaging (MRI) of the scrotum can also be considered. MRI has the advantages of a wider field of view and increased tissue contrast and spatial resolution compared to US. MRI is especially helpful in the characterization of intratesticular and paratesticular masses when a benign mass is likely.[10] Identification of fat, fluid, hemorrhage, or fibrous tissue on MRI may aid the diagnosis of sonographically indeterminate masses and prevent unnecessary surgical intervention. Further, MRI has shown to be correlated with histologic characteristics of malignant testicular tumors, allowing preoperative classification of the tumor type. Tsili et al. showed that MRI characteristics correlate with differentiation of histology-proven seminomatous and non-seminomatous testicular tumors.[11] Low signal intensity on T2-weighted enhanced images is more suggestive for the diagnosis of a seminoma, whereas tumors that are heterogeneous on unenhanced and contrast-enhanced images suggest a non-seminomatous lesion. When tumors of discordant histological type are suspected, MRI can be pursued to support this pre-operative diagnosis.
The presence of multiple testicular masses is an important consideration when treating testicular tumors, as multiple tumors may be a contraindication to testicular sparing surgery.[7] Identification of all tumor types present is critical, as cancer staging, treatment, and surveillance depend on the identification of the highest risk tumor type.[12]
CONCLUSION
In this case study, we have described a case of unilateral synchronous testicular tumors with distinct sonographic features and discordant histology of seminoma and mixed germ cell tumor, with the largest dimensions to be reported in literature. There are few reports on such tumors. Further studies to better understand the clinical impact of unilateral synchronous testicular tumors with discordant histology is an important aim of future research.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vikram Dogra is on the Editorial Board of the Journal.
References
- International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973-2007. Andrology. 2015;3:4-12.
- [CrossRef] [PubMed] [Google Scholar]
- From the archives of the AFIP: Tumors and tumorlike lesions of the testis: Radiologic-pathologic correlation. Radiographics. 2002;22:189-216.
- [CrossRef] [PubMed] [Google Scholar]
- The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. Eur Urol. 2016;70:93-105.
- [CrossRef] [PubMed] [Google Scholar]
- Best cases from the AFIP: Bilateral testicular tumors: Seminoma and mixed germ cell tumor. Radiographics. 2005;25:835-9.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral testicular germ cell tumors. Turk J Urol. 2013;39:249-52.
- [CrossRef] [PubMed] [Google Scholar]
- Synchronous bilateral testicular tumors with different histopathology. Case Rep Urol. 2015;2015:492183.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple, synchronous lesions of differing histology within the same testis: Ultrasonographic and pathologic correlations. Urology. 2018;121:125-31.
- [CrossRef] [PubMed] [Google Scholar]
- Histology and clinical outcomes in patients with bilateral testicular germ cell tumors: The memorial sloan kettering cancer center experience 1950 to 2001. J Urol. 2003;169:2122-5.
- [CrossRef] [PubMed] [Google Scholar]
- Bilateral testicular germ cell tumours: A systematic review. BJU Int. 2012;110:1102-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sonographically indeterminate scrotal masses: How MRI helps in characterization. Diagn Interv Radiol. 2018;24:225-36.
- [CrossRef] [PubMed] [Google Scholar]
- MRI in the histologic characterization of testicular neoplasms. AJR Am J Roentgenol. 2007;189:W331-7.
- [CrossRef] [PubMed] [Google Scholar]
- The approach to the patient with synchronous bilateral germ cell tumors: A lesson in oncologic prioritization. Oncology (Williston Park). 2010;24:761-3.
- [Google Scholar]







