Translate this page into:
Point of Care Neurosonogram in Neonates - Utility and Prognostic Value

Corresponding Author: Bhushita Lakhkar, Department of Radiology Shri B M Patil Medical College, Hospital and Research Center, Staff Quarter 22, South A Wing, SMT Bangaramma Saj, Bijapur–586 103, Karnataka, India. E-mail: bhushitalakhkar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Lakhkar B, Patil MM, Lakhkar B, Lakhkar B. Point of Care Neurosonogram in Neonates - Utility and Prognostic Values. Am J Sonogr 2019;2(1) 1-6.
Abstract
Objective
The study aimed to utilize the neurosonographic findings in neonates in early diagnosis, prediction of their long-term outcome, parental counseling, and early intervention.
Methods
The study was carried out in neonatal intensive care unit (NICU) of Shri BM Patil Medical College and Hospital. All preterms and term babies with neurological clinical findings were included in the study. Neurosonogram was done within first 7 days in preterms and when indicated in terms. Philips HD11XE ultrasound and color Doppler unit were used with a small footprint probe. Color Doppler images for vessels were performed for screening of vascular changes.
Results
A total of 215 babies were included, of which 80 (32%) were term and the rest were preterm. Mean weight of term babies was 2.8 kg and that of preterm was 1.2 kg.Among term babies, 78% showed ultrasound abnormality, and among preterm, 42%showed abnormalities. Among term babies, 60% and, among preterms, 30% had birth asphyxia. Periventricular leukomalacia was the most common and earliest finding followed by thalamic hyperechogenicity and intracranial hemorrhage. Intraventricular hemorrhage was more common in preterm babies. Other common finding in NICU was meningitis which was more common in pretrms. Among congenital anomalies, corpus callosal agenesis was more common.
Conclusions
Point of care ultrasonography along with Doppler study is very useful and safe to use in NICUs. It helps in diagnosis, patient management as well as prediction of many short- and long-term outcomes.
Keywords
Birth asphyxia
Intraventricular hemorrhage
Neurosonogram
Term and preterm babies
INTRODUCTION
New borns, specially pretem babies hsow non specific signs and symptoms if they are sick. Brain involvement of any kind in these babies may have long-term implications. Early diagnosis and prediction of long-term complications may help in effective parental counseling, early intervention, and better outcome.
Neurosonogram is a simple non-invasive, affordable investigation with no radiation exposure to patient. Fontanelle which is popularly called a “window to brain” can be utilized to view most pathologies occurring in newborn brain. If pathology like bleed is detected, serial follow-up can be done at the point of care of the baby affected. Hence, the point of care neurosonogram is becoming popular in neonatal intensive care unit (NICU).
The study aimed to utilize the neurosonographic findings in neonates in early diagnosis, prediction of their long-term outcome, parental counseling, and early intervention. This study was done in all preterms admitted in NICU and in term babies with neurological symptoms over a period of 1.5years.
METHODS
The study was carried out in NICU of Shri BM Patil Medical College and Hospital, Vijayapur, Karnataka, after obtaining ethical clearance from the Institutional Ethical Committee of BLDE University, Vijayapur. All preterm and term babies with neurological clinical features were recruited after obtaining consent from parents. Babies with parents’ denying consent were excluded from the study.
Neurosonogram was done within first 7 days in preterm (between 3 and 7th days) and when indicated in term babies. Indications in terms included the presence of seizures, lethargy, and asphyxia at birth and facial dysmorphology (which is usually associated with brain anomalies). Repeat scan was done if lesion was considered dynamic like intracranial bleed. The details of baby, diagnosis, and the changes in clinical course during NICU stay until death or discharge were entered in a pre-validated pro forma. Neurosonogram findings were entered in a separate structured format. Positive findings in neurosonogram were correlated with clinical status in the NICU, cause of death if baby died, or neurological status at discharge.
Babies were managed as per NICU protocol, and neurosonogram report was used to modify the treatment and counseling as required.
Equipment and procedural details
Philips HD11XE ultrasound and color Doppler unit were used. A high-frequency phased array transducer (5–8 MHz) with a small footprint probe was used for neurosonogram.
Neurosonogam was performed bedside with the neonate within the baby trolley. The patient was scanned generally through the anterior fontanel; at times, posterior fontanel and mastoid view were also used. Pressure over the anterior fontanel was avoided, especially in a critically ill, premature neonate. Initially, gray-scale imaging was performed through the anterior fontanel in the coronal and sagittal planes. In general, 6–8 coronal images were obtained, beginning at the anterior frontal lobes and extending to the occipital lobes, posterior to the lateral ventricle trigone. The transducer was then rotated 90°, and five sagittal images were obtained, including a midline and two parasagittal views of the right and left hemispheres encompassing the peripheral cortex. Findings such as intraventricular hemorrhages, germinal matrix hemorrhages, periventricular flare, and congenital anomalies were evaluated. Color Doppler images for arterial and venous structures were performed for screening of vascular changes. Spectral tracing with pulsatility index (PI) and resistive index (RI) of the middle cerebral artery and anterior cerebral artery was recorded for the evaluation of birth asphyxia.[1]
As NICU needs high-quality asepsis, handwash by the sonographer and disinfection of transducer were carefully followed.[2]
RESULTS
A total of 215 babies were included, of which 80 (32%) were term and the rest were preterm. Mean weight of term babies was 2.8 kg (range 1.8–3.1 kg) and 16 babies (20%) had intrauterine growth retardation. Mean weight of preterm babies was 1.2 kg (range 760 g–1.6 kg). Male-female ratio among preterms was 1.8:1, and among terms, it was 1.2:1.
Among term babies, 48 (60%) had hypoxic-ischemic encephalopathy following birth asphyxia, 15 (19%) had meningitis, and 6 (7.5%) had dysmorphology. There were 20 (25%) sick babies (i.e., babies suffering from birth asphyxia, respiratory distress syndrome (RDS), neonatal jaundice, and intraventricular bleed) but all survived (Table 1).
| Clinical symptoms | Term babies | Preterm babies |
|---|---|---|
| Birth asphyxia | 48 | 17 |
| Meningitis | 15 | 18 |
| Neonatal jaundice | 7 | 45 |
| RDS | 5 | 56 |
| Bulging anterior fontanelle | 5 | 7 |
| Sepsis | 0 | 66 |
RDS: Respiratory distress syndrome
Among 135 preterm babies, 54 (40%) were <32 weeks, 11 (8%) were <1 kg, and 45 (33%) were between 1 and 1.5 kg. 56 babies (41%) had RDS, of which 8 were ventilated. Sepsis was diagnosed in 66 (49%) babies, of whom 18 had meningitis, 17 babies had bleeding disorder, 45 (33%) babies had jaundice and needed phototherapy, and 4 needed exchange transfusion. Birth asphyxia was found in 17 babies. There were a total of 88 (65%) sick babies (babies with sepsis, RDS, neonatal jaundice, meningitis, etc), of which 11 (8%) died before discharge (Table 1).
Among term babies, 62 (78%) showed ultrasound abnormality as these babies had neurological clinical features (Table 2). In preterms, 42% had sonographic abnormalities. Among term babies, 60% and, among preterms, 30% had birth asphyxia. Various findings in birth asphyxia were periventricular flare (Figure 1), periventricular leukomalacia (PVL), thalamic hyperechogenicity, parenchymal hemorrhage, and cerebral edema. PVL (40%) was the most common (Figure 2) and earliest finding followed by thalamic hyperechogenicity (13%) and intracranial hemorrhage (10%). Cerebral edema with effacement of bilateral sulcogyral spaces was seen in 12% of babies who had birth asphyxia. Patients with intraventricular hemorrhage (IVH) had a poor prognosis (Figure 3). It was more common in preterm as compared to term babies. Four of these babies had extension of intraventricular hemorrhage into cerebral parenchyma. Parenchymal bleed was again more common in preterm as compared to term babies.
| Neurosonographic findings | Term babies (n=80) (%) | Preterm babies (n=135) (%) |
|---|---|---|
| Suggestive of birth asphyxia | 33 (41)** | 10 (7) |
| Periventricular/parenchymal hemorrhage | 06 (8) | 16 (12) |
| Evidence of meningitis | 10 (13) | 12 (9) |
| Hydrocephalous communicating/obstructive | 5 (4) | 4 (3) |
| Choroid plexus cyst | 2 (1) | 1 (0.7) |
| Congenital anomalies | 5 (4) | 9 (7) |
| Abnormal doppler findings | 11 (8) | 5 (4) |
| Total babies affected | *62 (78) | 57 (42) |
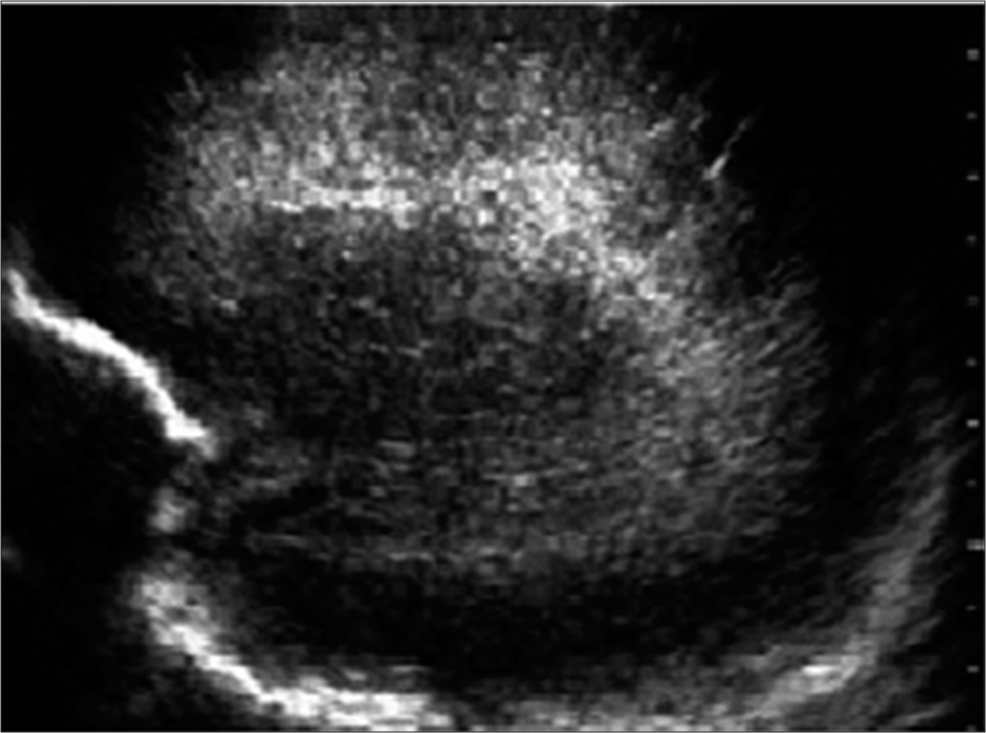
- Periventricular flare: Sagittal image through anterior fonatnelle in a 2-day-old term baby showing hyperechogenicity surrounding the lateral ventricles.
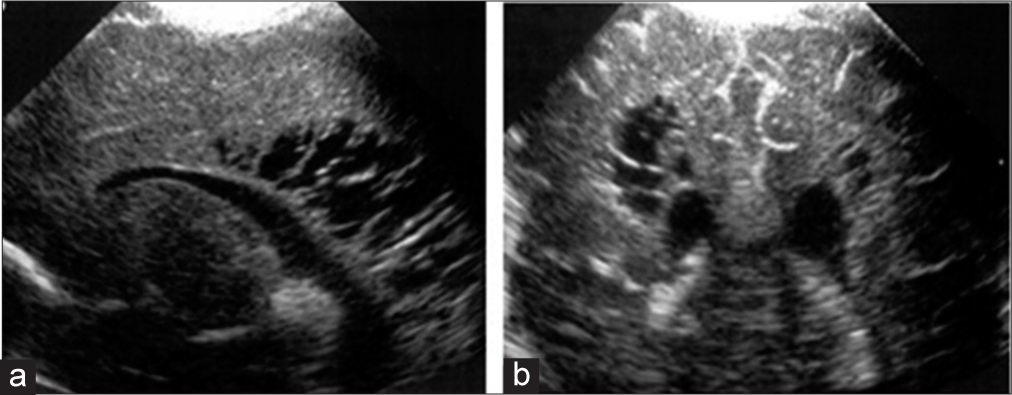
- Periventricular leukomalacia with cystic encephalomalacia: Coronal image (a) and saggital (b) through anterior fonatnelle in a preterm baby show ill-defined hyperechogenicity in periventricular region with few cystic changes noted.
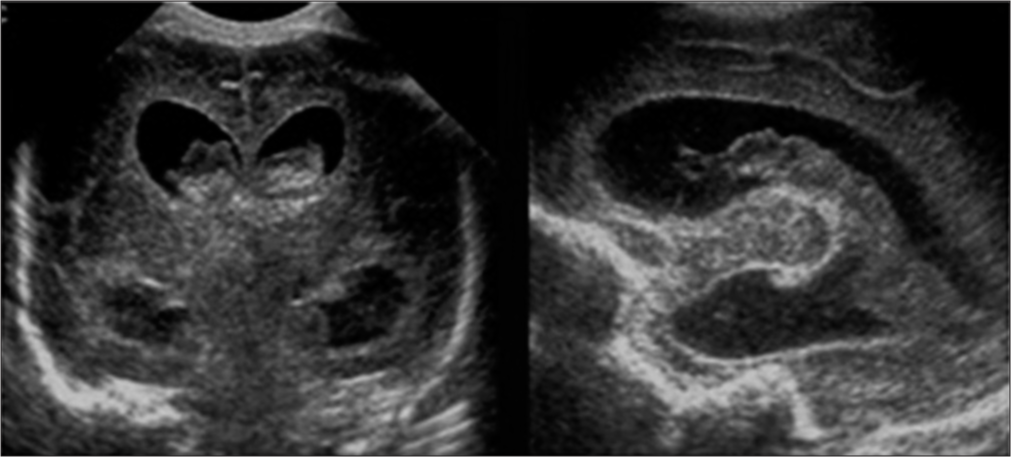
- Intraventricular hemorrhage (Grade III): Sagittal and coronal image through anterior fontanelle in a 6-day-old term baby showing hyperechogenic bleed within bilateral lateral ventricles with dilatation of the ventricles.
Next to birth asphyxia, meningitis was a common finding. It was seen in 12 preterm and 10 term babies. Few patients of meningitis also showed signs of the effacement of sulcogyral spaces, indicating cerebral edema. Five cases of meningitis had communicating hydrocephalus.
Among congenital anomalies, most common was complete and partial corpus callosum agenesis (Figure 4). Other anomalies were Dandy–Walker malformation and hydrocephalus due to aqueductal stenosis.
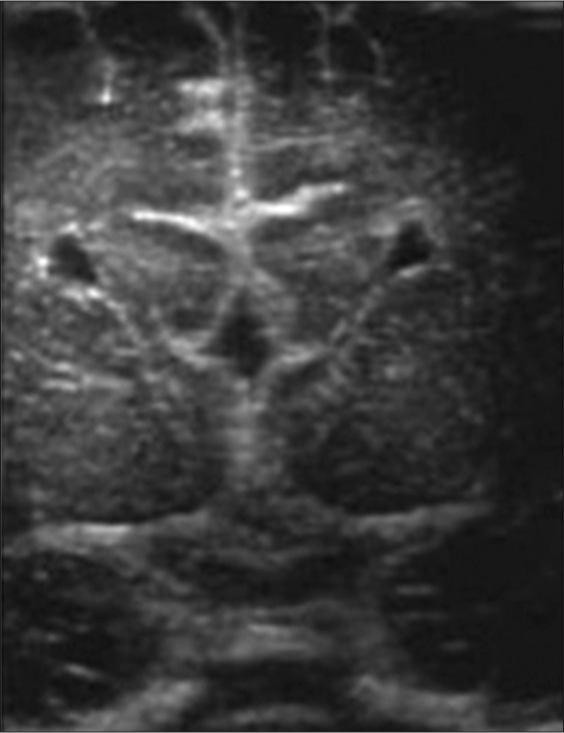
- Corpus callosal agenesis-viking horn appearance: Coronal image of a term baby through anterior fontanelle showing absent genu and splenium of corpus callosum giving a typical viking horn appearance.
Few babies presented with miscellaneous findings such as choroid plexus cyst and mega cisterna magna (Figure 5).
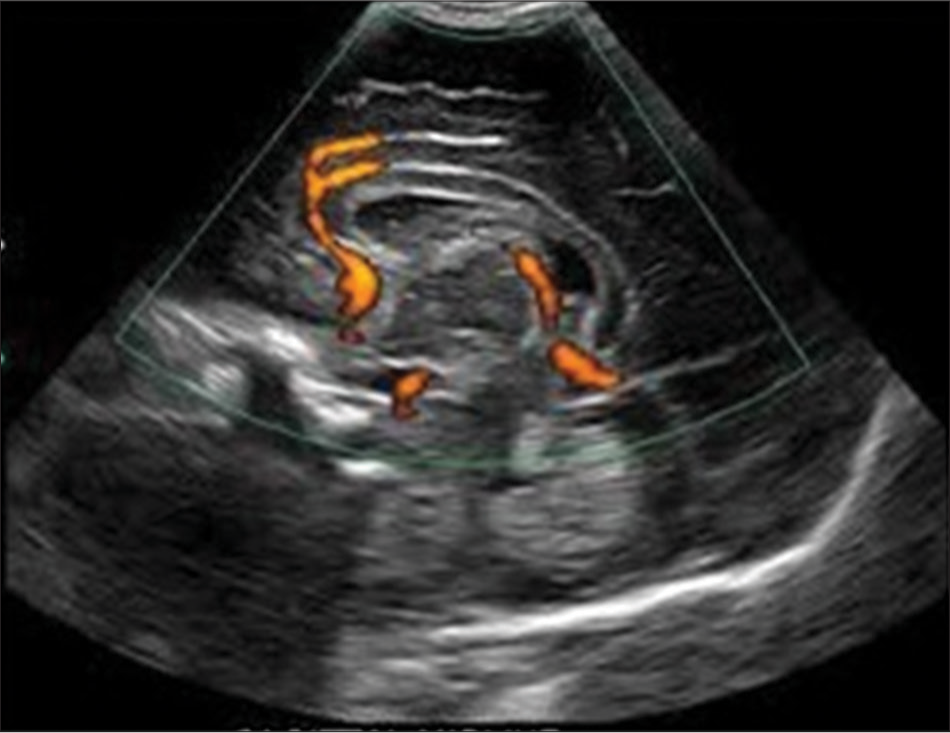
- Neurosonogram saggital section through anterior fontanelle showing normal anterior cerebral artery around corpus callosu
Doppler was done in all the babies, and PI and RI were calculated in anterior cerebral arteries and middle cerebral arteries. Normal value of RI was considered to be between 0.56 and 0.80[3] and that of PI 1.1 and 1.14. Anything above or below these values was considered abnormal. A total of 15 patients showed abnormal Doppler values, of which 11 were term babies and 5 were preterm, and these babies had their RI value >0.80, which indicated cerebral vascular insult and correlated with birth asphyxia.
Sonographic abnormalities were more common <32 weeks, <1.5 kg, and in sick babies. Among 11 patients who died, 9 (82%) had abnormalities (Table 3).
| Risk factors | Sonographic abnormality | p value |
|---|---|---|
| Gestational age (weeks) | ||
| <32 | 21 (40) | 0.01 |
| >32 | 17 (21) | |
| Weight (Kg) | ||
| <1.5 | 27 (48) | 0.0006 |
| >1.5 | 16 (20) | |
| Sick babies | ||
| Yes | 46 (41) | <0.0001 |
| No | 5 (10) | |
| Jaundice | ||
| Yes | 2 (5) | |
| No | 0 (0) |
Serial ultrasonography was done in cases of hemorrhage and hydrocephalus. 3 patients that showed rapid increase in haemorrhage died. In these cases, parents could be counseled for poor outcome. Hydrocephalus in four cases was arrested and one developed ventriculitis, and poor prognosis could be predicted. Thalamic changes and PVL in birth asphyxia, presence of anomalies, and Doppler changes were useful to predict poor outcome. Parental counseling for early intervention and good follow-up was possible Cerebral edema detection was helpful in management.
DISCUSSION
Point of care ultrasonography done routinely in preterm and when indicated in term babies is very helpful in managing and predicting the outcome, which, in turn, is useful for parent counseling. We found a significant correlation of the presence of abnormal ultrasonographic findings with gestational age <32 weeks, weight <1.5 kg, birth asphyxia, sepsis, and RDS. Nagaraj et al.[4] also noted similar observation. They found a correlation with jaundice also which was not observed by us. PVL mainly indicates cerebrovascular insult. It occurs due to chronic hypoxic changes, hence common in birth asphyxia, especially, in term and also low birth weight babies. Few patients of PVL on follow-up studies also showed the presence of cystic encephalomalacic changes within the brain parenchyma. Patients with Grade III and IV changes of birth asphyxia are specially reported to be associated with poor long-term prognosis.[5] Birth asphyxia might also present with cerebral edema neurosonographically. We found it in 12% of the cases. Prithviraj et al. found similar findings.[6] Diagnosing cerebral edema is still a challenge on sonography, and magnetic resonance imaging (MRI) remains a better modality,[7] but if noticed it is useful in management.
In ischemic lesions, Doppler study, especially diastolic flow measured as restrictive index, is useful. We found that serial values are more useful, and Mack et al. also noticed this.[8]
Porencephalic cysts which indicate old Grade IV hemorrhage were not observed by us but when present indicate poor long-term outcome, and neurodeficits are predictable.[9] Choroid plexus cyst is common and harmless until and unless it obstructs cerebrovascular fluid pathway. In our case, it was non-obstructive.
Grade I and II germinal matrix bleed usually is not associated with long-term sequel; the chances increase with increasing severity of bleed and ventricular dilation.[10] We found high-grade bleed (Grade III) in two babies <1000 g and both died. Higher grade, especially Grade IV bleed, can cause hydrocephalus which can be diagnosed on follow-up ultrasound.[11,12] Subarachnoid and subdural bleed is not visualized by neurosonogram. The use of high-frequency linear transducer can help in subdural bleed.[13] In our study, 10% of the patients had IVH, of which 4 had extension into cerebral parenchyma. It was more in preterm babies. Similar finding was observed by Humsene et al. who found IVH in 13% of the cases and was more in preterms.[14] Other published studies also correlated the degree of prematurity and the increased ICH risk,[15,16] Hence, it is generally accepted that the incidence and severity of the ICH are related to both gestational age and birth weight, occurring in 25–30% of patients born at <32 weeks gestation, with <1500 g.[14,15]
Diagnosis of meningitis on sonography is particularly useful for clinicians, especially when lumbar puncture is not possible due to poor general condition of patients. Stepping up of antibiotics and higher doses for longer duration are needed in meningitis. Pyogenic meningitis was present in 22 babies, more in preterm. Ultrasonographically, there was the presence of dense echogenic sulci or meningeal thickening at few places. At times, cerebral edema may also be seen. These changes are better depicted on computer tomography or MRI. Similar findings were seen in a study by Humsene et al.[14] Association of ventriculitis and communicating hydrocephalous with meningitis indicates a poor prognosis. In the present study, five cases of meningitis had communicating hydrocephalus.
Corpus callosal agenesis was the most common anomaly and the same is described by other authors. Usually has a good prognosis though seizures and delay in development may be there.[17] MRI should be done in these children at a later date to confirm partial or complete agenesis and other associated anomalies.[18]
Calcifications if seen are a hallmark of TORCH group of infections and poor prognosis.[19] Doppler study was done to see restrictive indices in anterior and middle cerebral arteries and is helpful in predicting prolong ischemia or intracranial hemorrhage or edemaalso noticed by other authors.[20] Normal velocity curves have been developed by Allison et al.[21]
Although cranial ultrasound is extremely useful in predicting problems in neonates and is also helpful in management, there are some limitations. Posterior fossa and posterior convex part of the brain are not well visualized. Three-dimensional (3D) high-frequency probe can be used in these situations but may not be available in the facility.[22] In ischemic lesions in the first 24 h, there are no sonographic changes. It is difficult to detect migration disorders such as cortical dysplasias, and cystic white matter lesions are better visualized than non-cystic ones which can be missed. It has a limitation of operator dependency also, which can be reduced if 3D US is available.[22,23]
CONCLUSIONS
Point of care ultrasonography along with Doppler study is very useful and safe to use in NICUs. It helps in diagnosis, patient management as well as prediction of many short- and long-term outcomes which is useful for patient counseling and early intervention. Availability of 3D US will further enhance the accuracy and utility.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- State-of-the-art cranial sonography: Part 1, modern techniques and image interpretation. AJR Am J Roentgenol. 2011;196:1028-33.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasonography of the Prenatal and Postnatal Brain (2nd ed.). New York: McGraw-Hill; 2010. p. :28-33.
- Variability of resistive indices in the anterior cerebral artery during fontanel compression in preterm and term neonates measured by transcranial duplex sonography. J Perinatol. 2014;34:306-10.
- [CrossRef] [PubMed] [Google Scholar]
- A study of neurosonogram abnormalities, clinical correlation with neurosonogram findings, and immediate outcome of high-risk neonates in neonatal intensive care unit. J Pediatr Neurosci. 2016;11:200-5.
- [CrossRef] [PubMed] [Google Scholar]
- Duration of transient hyperechoic images of white matter in very-low-birthweight infants: A proposed classification. Dev Med Child Neurol. 1997;39:2-5.
- [Google Scholar]
- Neurosonogram in critically ill neonates in neonatal intensive care unit. Int J Sci Stud. 2016;4:124-8.
- [Google Scholar]
- MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics. 2000;31:128-36.
- [CrossRef] [PubMed] [Google Scholar]
- Intracranial hemorrhage in premature infants: Accuracy in sonographic evaluation. AJR Am J Roentgenol. 1981;137:245-50.
- [CrossRef] [PubMed] [Google Scholar]
- Practice parameter: Neuroimaging of the neonate: Report of the quality standards subcommittee of the American academy of neurology and the practice committee of the child neurology society. Neurology. 2002;58:1726-38.
- [CrossRef] [PubMed] [Google Scholar]
- The resuscitation and care of the newborn at risk. In: DeCherney AH, Nathan L, Goodwin TM, Laufer N, eds. Current Diagnosis and Treatment: Obstetrics and Gynecology (10th ed.). New York: McGraw Hill; 2007.
- [Google Scholar]
- Pediatric Sonography (3rd ed.). Philadelphia, PA: Lippincott Williams and Wilkins; 2001.
- Neonatal Neurology (4th ed.). Philadelphia, PA: Saunders; 2001.
- High-frequency linear array transducers for neonatal cerebral sonography. AJR Am J Roentgenol. 2001;176:995-1001.
- [CrossRef] [PubMed] [Google Scholar]
- Transcranial perinatal neurosonogram for intracranial pathology: Study of 165 cases. J Med Sci Health. 2016;2:29-36.
- [Google Scholar]
- Risk factors for intraventricular hemorrhage in very low birth weight premature infants: A retrospective casecontrol study. Pediatrics. 2003;111:e590-5.
- [CrossRef] [Google Scholar]
- Risk factors for intraventricular hemorrhage in a birth cohort of 3721 premature infants. J Perinat Med. 2000;28:104-10.
- [CrossRef] [PubMed] [Google Scholar]
- Agenesis of the corpus callosum: Neonatal sonographic detection. Radiol Technol. 2006;78:14-8.
- [Google Scholar]
- Neonatal and infant brain imaging. In: Rumack CM, Wilson SR, Johnson JA, Charboneau JW, eds. Diagnostic Ultrasound (3rd ed.). St. Louis: Elsevier Mosby; 2005. p. :1623.
- [Google Scholar]
- Neonatal cranial sonography: Moderns strategies and applications. Diagn Imaging Contin Med Educ. 2007;2017:1-6.
- [Google Scholar]
- Intracranial resistive index (RI) values in normal term infants during the first day of life. Pediatr Radiol. 2000;30:618-20.
- [CrossRef] [PubMed] [Google Scholar]
- Three-dimensional ultrasonographic imaging of the neonatal brain in high-risk neonates: Preliminary study. J Ultrasound Med. 2000;19:549-55.
- [CrossRef] [PubMed] [Google Scholar]
- Multiplanar 3-dimensional neonatal neurosonography: Initial experiences and potential benefits. J Diagn Med Sonogr. 2001;17:3-13.
- [CrossRef] [Google Scholar]







