Translate this page into:
Reversal of Blood Flow in the Internal Jugular Vein - A Case Series and Review of the Literature

Corresponding Author: Akshaar Brahmbhatt, Department of Imaging Sciences, 601 Elmwood Avenue, Box 648, Rochester, New York, United States, 14642. E-mail: akshaar@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Brahmbhatt A, Miceli R, Skalski K, Dogra V. Reversal of Blood Flow in the Internal Jugular Vein - A Case Series and Review of the Literature. Am J Sonogr 2018;1(17) 1-7.
Abstract
Objective
The objectives of this study were to identify the causes of internal jugular vein (IJV) blood flow reversal revealed on ultrasound imaging.
Methods
In this retrospective review, 4796 upper extremity venous ultrasounds completed at a single institution between January 2012 and December 2017 were reviewed to identify cases of flow reversal in the IJV. Fourteen patients were identified with IJV flow reversal. Medical charts of these 14 patients were reviewed to identify the etiology of blood flow reversal.
Results
Intraluminal causes were the most common and were most frequently seen in patients with vascular damage secondary to placement of endovascular devices. Flow reversal most commonly occurred in the left IJV and was equally represented in men and women. Ages ranged from 41.38 to 82.76 years, with an average age of 61.92 years.
Conclusion
Reversal of flow in the IJV is a rare finding which is most often diagnosed on ultrasound evaluation of the upper extremity. Further investigation should be performed when flow reversal is identified, as the underlying cause may have serious clinical implications.
Keywords
Flow reversal
Internal jugular vein
Vascular ultrasound
Superior vena cava syndrome
Central venous stenosis
INTRODUCTION
Reversal of blood flow in the internal jugular vein (IJV) is an uncommon imaging finding. Prior case reports have described a variety of causes.[1-4] These etiologies can be divided into three main categories, which can occur in isolation or in combination, resulting in IJV flow reversal. Intraluminal and mural causes include venous occlusion or partial obstruction from stricture, stenosis, thrombosis, surgical ligation, or intimal injury. Extrinsic causes include extrinsic compression from adjacent tumor, aneurysm, or lymphadenopathy. Finally, altered hemodynamics, commonly caused by arteriovenous fistulas (AVFs), can result in flow reversal.
Reversal of IJV blood flow is clinically significant because it implies that there is more central venous pathology, such as stricture, stenosis, or mass. Therefore, the discovery of this finding warrants appropriate patient triage and management. There is diverse range of the causes of IJV flow reversal. This study aims to provide better insight into the prevalence, causes, and imaging findings of IJV flow reversal.
Flow reversal in the IJV is a rare finding discovered on ultrasound (US) using color flow Doppler (CFD) to rule out deep venous thrombosis (DVT) of the upper extremity (UE). Doppler US is an indispensable technique used in the evaluation of vascular structures and pathology. US is an advantageous modality because it is non-invasive, real-time, easily accessible, portable, inexpensive and does not require contrast administration. Nonetheless, the gold standard for diagnosis of flow reversal is still conventional contrast venography. However, this is an invasive test reserved for patients with known pathology or those undergoing intervention.
Etiologies
A diverse range of causes of IJV flow reversal has been previously described in the literature using CFD imaging.[4-8] The most common cause is a central occlusion in the lower IJV, brachiocephalic vein, or superior vena cava (SVC). Occlusion can occur due to thrombus or stenosis in these vessels. Flow reversal can also occur due to the placement of an UE dialysis fistula, which is often associated with a central stenosis. Central stenosis can occur due to intimal injury from intravascular devices and instrumentation. It can also occur due to radiation or an extrinsic compression from a mass. De novo thrombus can also result from hypercoagulable states, such as malignancy.[4] For the purpose of this review, causes were broken down into three categories (Table 1).
| Intraluminal and mural | Mural injury |
| Vasculitis | |
| Thrombosis | |
| Fibrosis | |
| Flow alteration | Arteriovenous fistula |
| Arteriovenous graft | |
| Internal jugular – carotid fistula | |
| Extrinsic | Mass |
| Aneurysm |
IJV: Internal jugular vein
Methods
A total of 4796 US reports for right, left, and bilateral UE venous US studies performed between January 2012 and December 2017 were reviewed. Inclusion criteria for flow reversal in the IJV were met if the final US report used the term(s) “reverse,” “reversal,” “reversed,” and/or “retrograde,” to characterize the flow of the left or right IJV. Patients were excluded if the study was incomplete or were younger than 18 years of age. We ultimately identified 14 cases which met our criteria. The patient charts, including notes and additional imaging tests, were then reviewed to determine associated symptoms and the most likely cause of blood flow reversal in the IJV. Patient gender, age, laterality of reversed IJV blood flow, history of malignancy, and the presence of catheters, pacemakers, and AVF were recorded.
This study protocol was reviewed and approved by the University of Rochester Research Subjects Review Board.
Image findings on US
Utilization of CFD as first-line imaging for obstruction makes it possible to assess the veins of the neck, UE, and thoracic inlet with relative ease. IJV flow is best evaluated from the angle of the mandible inferiorly to the central vessels using a 5–7 MHz linear phase array. The inferior IJV, innominate, and subclavian veins can be evaluated with a small footprint transducer such as a 2–4 MHz phase array probe. It is important to visualize around the clavicle, which can limit the evaluation.[9] Curvilinear probes can be used to examine central vessels from a suprasternal approach.[10] An additional tool is vector flow imaging. These techniques can help to overcome angle dependence and aliasing. This latter technique can also be aid in identifying and evaluating tortuous collaterals and complex flow patterns.[11,12]
Normal flow in the IJV directionally opposes the nearby carotid artery and is usually blue by convention, as the transducer is angled toward the head. The normal venous pattern is biphasic and beings with an upward deflection “a,” which corresponds to the atrial contraction. This is followed by the first small descent, which corresponds to the pulling of the right atrium at the end of ventricular systole. This is followed by “c” likely a result of tricuspid or carotid impulse transmission. This is followed by a larger downward “x” deflection followed by an upward “v” which corresponds to ventricular systole and finally a downward “y” deflection during passive ventricular filling.[13] The pattern can be highly variable due to patients’ position as well as their cardiovascular, pulmonary status, and volume status among others. In such cases, evaluation of the contralateral side can provide an internal control. Major alterations usually occur due to cardiac abnormalities and intermittent reflux are often present with congestive heart failure.[14] These transient reversals or reflux is in contrast to flow reversal, which is persistently reversed (Figure 1a).
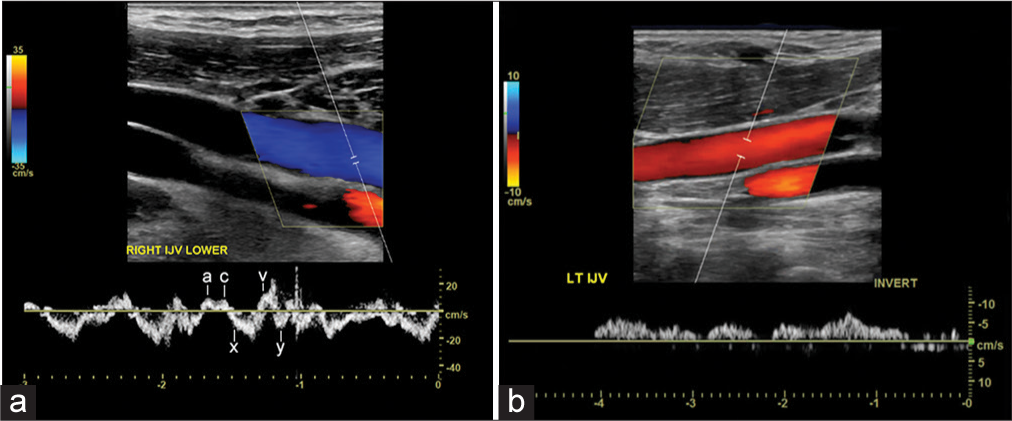
- (a) 27-year-old male with normal upper extremity venous flow. Major deflections in the waveform are noted. (b) 54-year-old female with left internal jugular (IJ) flow reversal with suspected left braciocephalic thrombus. Spectral ultrasound demonstrates reversal of flow in the left internal jugular (arrow) as the direction of flow is idential to that of carotid artery (white arrow head). Note the irregular venous waveform.
Reversal of flow can be denoted on CFD if there is flow in the same direction of both the carotid and jugular, barring any arterial pathology (Figure 1b)[15-17]. If there is a reversal of flow, it is important to search for several associated findings. The most common is central venous thrombus, which is sonographically identified by a lack of luminal compressibility, intraluminal echogenic material, and/or the absence of flow in a more central vein. If these are not visualized, one should check for more peripheral dilated collateral veins or no change in the spectral tracing with respirations and Valsalva maneuver. Both of these findings suggest central thrombosis or stenosis. Venous stenosis should be suspected if there is increased flow velocity and turbulence. Comparison should be made with the contralateral side.[15-17]
RESULTS
Fourteen patients were identified with IJV flow reversal (Table 2). Flow reversal most commonly occurred in the left IJV and was equally represented in men and women. Intraluminal and mural causes were the most common cause seen in 57.1% of patients. Most of these were attributed to a history of endovascular device placement. The second most common group comprised of patients with a history of AVF, 35.7%. Finally, a single patient was found to have flow reversal due to an extrinsic mass. Patients were most commonly presented with a unilateral UE edema along with several other associated symptoms (Table 3). Several patients presented with multiple symptoms.
| Cause | n(%) | Gender (%) | Laterality (%) | Etiology |
|---|---|---|---|---|
| Intraluminal and mural | n=8 (57.1) | Male=4/8 (50) | Right=1/8 (12.5) | Central venous catheter: 4 |
| Pacemaker: 1 | ||||
| Malignancy: 3 | ||||
| Hemodynamic shift | n=5 (35.7) | Male 3/5 (60) | Right 1/5 (20) | AVF: 5 |
| Extrinsic | n=1 (7.1) | Male 1/1 (100) | Right 1/1 (100) | Mass: 1 |
AVF: Arteriovenous fistulas
| Associated symptoms | Number of patients |
|---|---|
| Unilateral UE edema | 10 |
| Shortness of breath | 2 |
| Neck fullness | 1 |
| Bilateral UE edema | 1 |
| Hypoxia | 1 |
| Chest pain | 1 |
| Shoulder pain | 1 |
| Facial pain | 1 |
| Jaw pain | 1 |
| Headache | 1 |
| Increased intracranial pressure | 1 |
| Lightheadedness | 1 |
| Palpable mass | 1 |
UE: Upper extremity
DISCUSSION
There have been a few case reports examining flow reversal in the IJV, but there was no systematic review to evaluate the causes for the IJV reversal of blood flow. Although jugular venous reflux can be seen with heart failure, continuous flow reversal is a much rarer incidental finding. In our review, we only found 14 cases on routine US to exclude DVT in the UE over a 5-year period. The majority of these were due to iatrogenic endovascular instrumentation and device placement, namely catheters and pacer leads (Figure 2). These often thrombogenic devices can induce stenosis from intimal injury during positioning and secondary to long-standing pressure on the intima. This inflammation then results in a cascade of fibrosis and eventual thrombus and stenosis (Figure 3). Similar molecular mechanism is seen in radiation-induced fibrosis.[18] This process can be exacerbated by lead or line infection. In addition, SVC syndrome has been shown to occur as early as 2 days after lead placement or several months later.[7] This lack of temporal relationship suggests that venous catheters and venous pacemaker leads can lead to central venous narrowing at any point in their use.
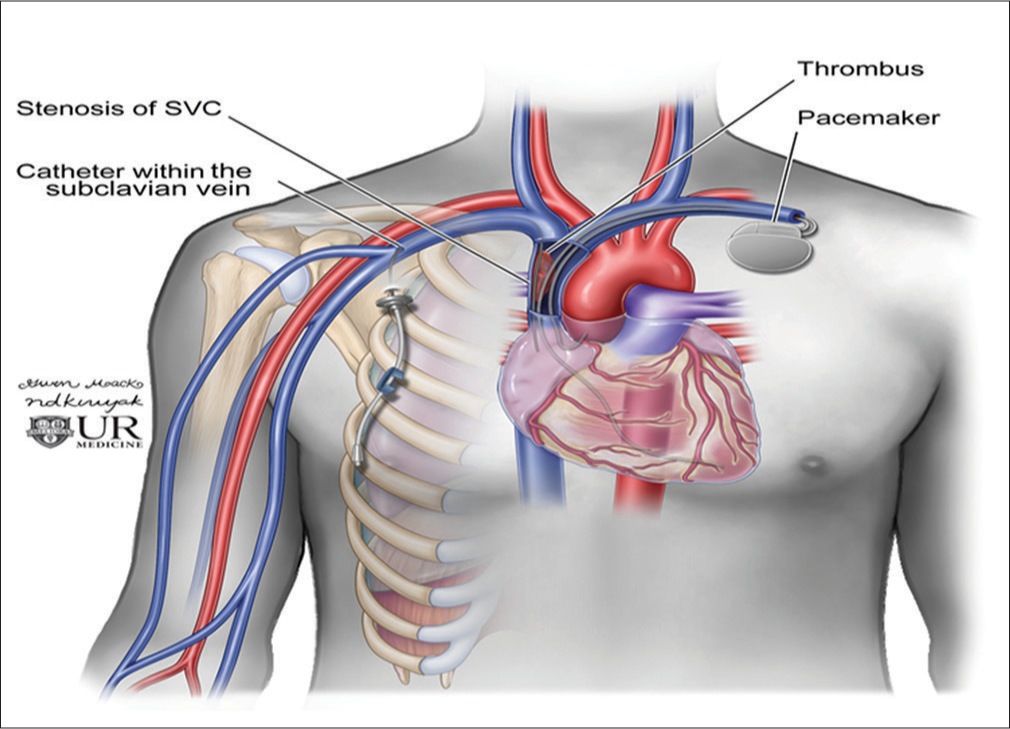
- Diagram of a cardiac pacer with wires coursing through the central veins and a right-sided subclavian catheter terminating in the superior vena cava.
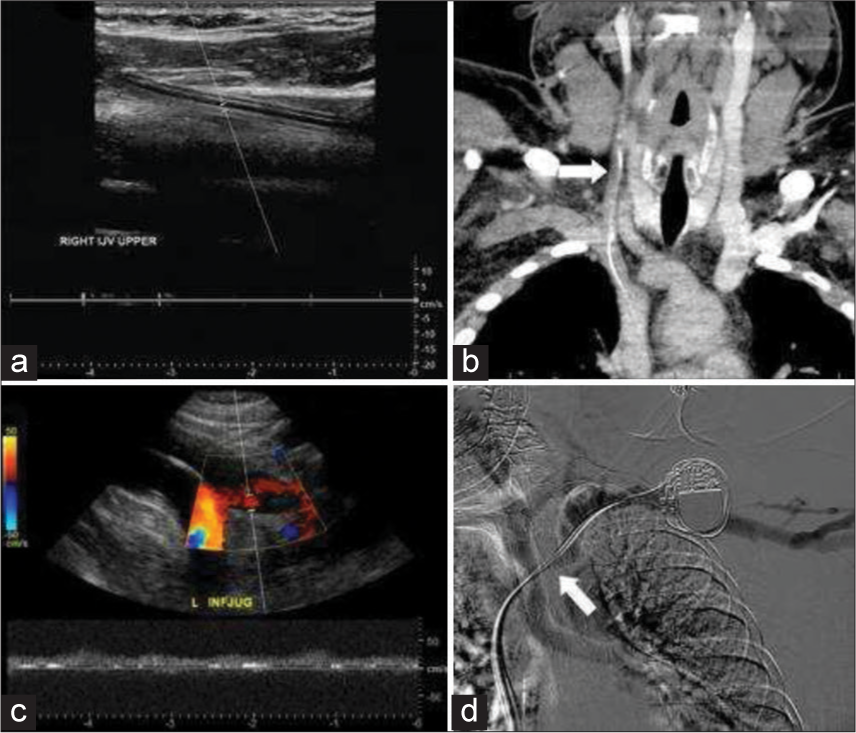
- Examples if intraluminal devices resulting in thrombus and stenosis. 41-year-old male with indwelling right internal jugular catheter (a and b). (a) Spectral Doppler demonstrates no flow within the right internal jugular vein (IJV) secondary to pericatheter thrombus. (b) Coronal Computer tomography demonstrates thrombus in the right internal jugular secondary to pericatheter thrombus (white arrow). 78-year-old female with left-sided cardiac pace maker (c and d) (c) real time color and spectral Doppler demonstrates occlusion of the left IJV. (d) Venogram demonstrates filling defect in the proximal left subclavian vein secondary to stenosis and thrombus around the cardiac device leads (white arrow).
Beyond direct intimal injuries, vessel wall abnormalities are also known to occur due to vasculitis, namely Behçet’s disease. Although rare, Behçet’s disease should be considered in younger symptomatic patients with vessel wall thickening, especially if they are without other known or suspected pathology.[19]
Beyond intraluminal and mural factors, flow alterations were the second most common cause (Figure 4). Hemodialysis AVFs function by progressively arterializing the draining vein. However, this process can go awry due to changes in shear stress, which can lead to neointimal hyperplasia, central stenosis, and ultimately occlusion (Figure 5). In addition, many hemodialysis patients have had central venous catheters or central venous instrumentation during fistulogram. This endovascular manipulation can lead to and exacerbate central stenosis.[20] Although rare, these patients are also at risk for developing reversal of flow due to iatrogenic fistulae created during catheter placement and central venous instrumentation.[2,21]

- Diagram of flow alteration due to an arteriovenous fistula and central stenosis.
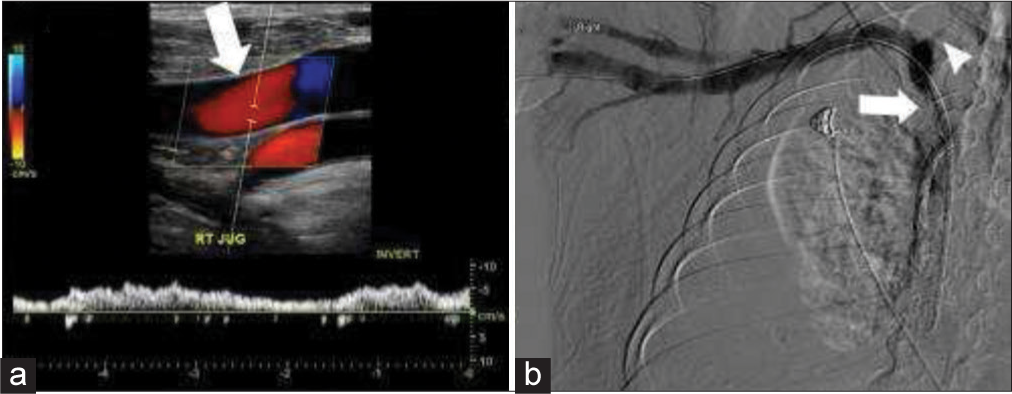
- 58-year-old male with right upper extremity arteriovenous fistula and central stenosis. (a) Real-time color and spectral Doppler demonstrate reversal of flow in the right internal jugular vein (IJV) (white arrow). (b) Venogram demonstrates central stenosis (white arrow) with reflux into a narrowed right IJV (white arrow head).
Extrinsic compression due to metastasis was identified as a cause of reversal of blood flow in the IJV in one patient (Figure 6). Although we found only one patient in our series, extrinsic compression is an important consideration. Extrinsic compression is the most common cause of SVC syndrome in the literature. Extrinsic compression of the central veins is most often due to a neoplastic process, namely lung cancer and lymphoma (Figure 7). Metastasis should be considered in a patient with a known malignancy. One should also consider non-neoplastic masses such as retrosternal goiter, aneurysm, or sclerosing mediastinitis as part of the differential.[22,23]
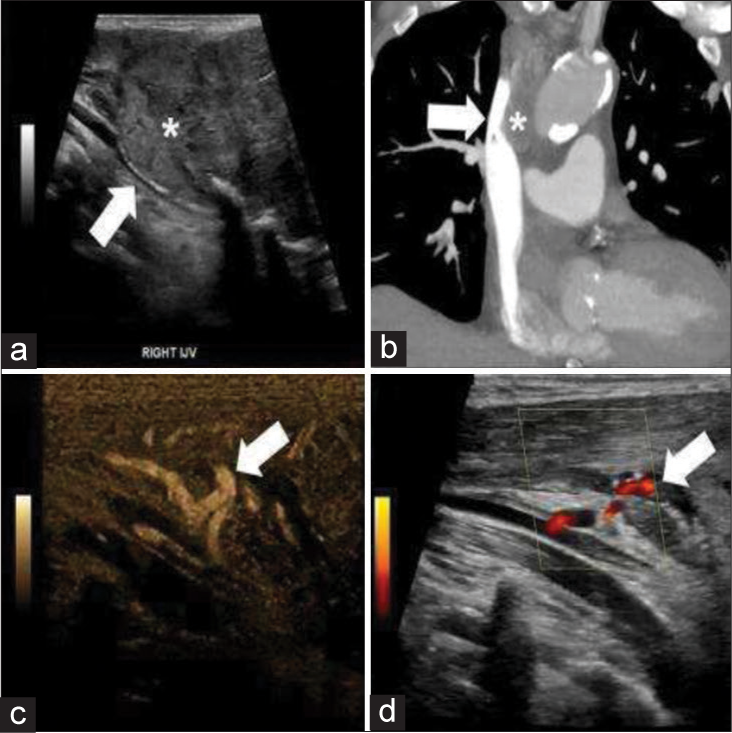
- 63-year-old male with metastatic renal cell carcinoma chronic extrinsic venous compression with resultant collateral formation. (a) Grayscale ultrasound demonstrates mass (asterisk) with compression of the right internal jugular (white arrow). (b) Coronal computed tomograhpy shows compression of the superior vena cava (white arrow) secondary to a surrounding mass (asterisk). (c) B flow images demonstrate flow in several collateral vessels (white arrows). (d) Power Doppler images demonstrate flow in several collateral vessels (white arrows).
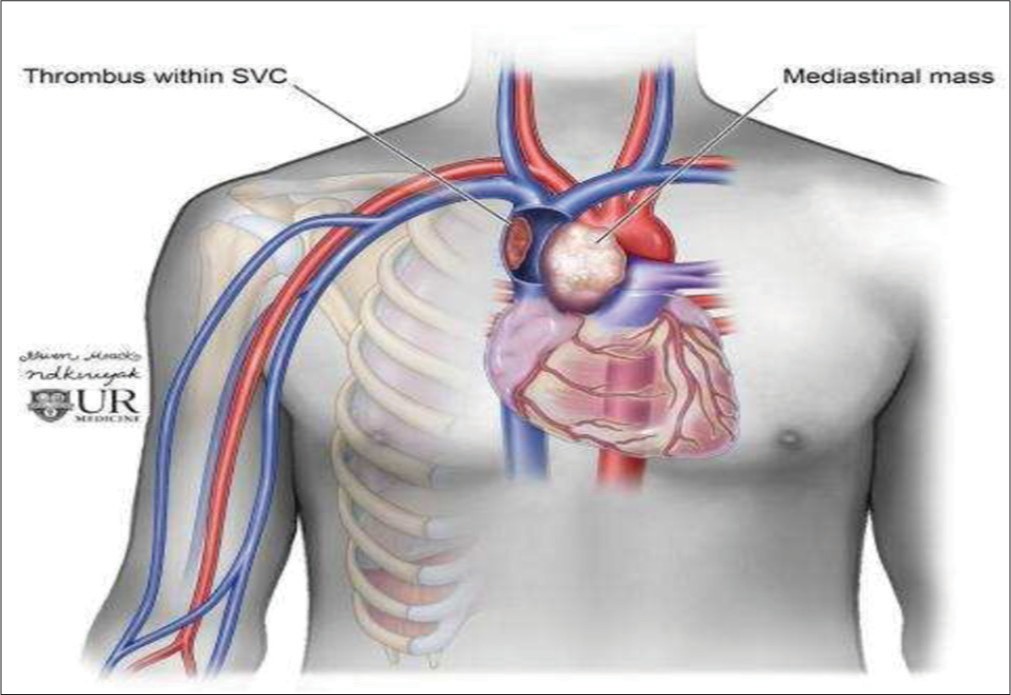
- Diagram depicting mediastinal mass resulting in extrinsic compression superior vena cava.
These cases of IJV flow reversal were found on UE venous US studies to exclude DVT, most patients presented with symptoms suspicious for UE DVT, and most commonly unilateral UE edema. Other less frequent symptoms included pain, shortness of breath, and headache. Multiple patients reported a number of symptoms.
Obstruction of the central veins has a spectrum of clinical implications and associated morbidity. While edema is relatively benign, other consequences such as alterations in cerebral blood flow, malignancy, and pulmonary embolism in the setting of thrombosis are associated with higher morbidity.[24] Due to these potential implications, it is important to recognize reversal of IJV blood flow.
In the case of IJV thrombosis, the most common complications are pulmonary embolism and post-thrombotic syndrome.[25] Reversal of flow in the IJV has also been linked to several neurological conditions, including transient monocular blindness and transient global amnesia, although the exact pathophysiology of these conditions remains unclear.[3,26] Cerebral venous thrombosis is another less common consequence of impaired venous outflow but is a significant source of morbidity. Cerebral venous thrombosis is considerably burdensome to patients, as it can present with severe symptom. In addition, it is difficult to diagnose, often requiring an extensive invasive workup and possible surgical treatment.[27]
Patients with flow reversal may also present with retropharyngeal edema. This has been documented in patients with IJV thrombosis and is thought to be due to intestinal edema or fluid from engorgement of the posterior pharyngeal venous plexus.[28] As an aside, a venous pathology should be excluded in patients with retropharyngeal edema as this may provide an etiology and prevent misdiagnosis.
Intervention following the discovery of flow reversal in the IJV is, therefore, targeted at the underlying cause. Thrombosis of the central veins is managed with anticoagulation. If the thrombus is associated with an indwelling catheter, then the line should be removed if feasible. The American College of Chest Physicians recommends 3 months of anticoagulation for patients with an UE DVT.[29] Thrombolysis may be considered if the patient is experiencing symptoms that interfere with daily life and is within 2 weeks of symptomatic onset.[30] If the flow reversal is due to extrinsic compression, anatomic abnormalities of the vessel, or persistent symptomatic thrombus, one should consider venoplasty, stenting, and surgical interventions.[31]
In cases of long-standing flow reversal or stenosis, one can expect the formation of collateral flow at multiple levels. Superiorly, there can be engorgement and collateral formation involving the thyroid branches, anterior jugular veins, and the jugular venous arch. Although less likely, reversal of flow may also lead to cranial condylar collaterals and alterations in the pterygoid venous plexus.[32,33] In addition to these collaterals, it is likely that the contralateral venous return will bypass the SVC entirely. In cases of more proximal stenosis or blockage, venous blood can travel through the azygous/accessory hemizygous - hemizygous system. Blood can also make its way through the vertebral and subscapular plexuses, ultimately draining into the lumbar veins. Mediastinal, esophageal, and pericardial collaterals can drain into the inferior phrenic or transhepatic branches. Lastly, collaterals can also form to the thoracic veins eventually draining to the thoracoepigastric or superficial epigastric veins. All of these collaterals will ultimately drain into inferior vena cava.[34]
Our review has several limitations. This review examined USs performed at a single tertiary care center, limiting the generalizability of these findings, as other centers are likely to have a differing etiological composite. Given the retrospective review of this study, some clinical details were not available for all patients. In addition, patients were not directly questioned for all possible associated symptoms at the time of US, thus limiting evaluation of associated symptoms.
CONCLUSION
Flow reversal in the IJV is a rare finding on US examination of the veins. In our review, flow reversal occurred most commonly due to central venous stenosis after endovascular device placement and intraluminal instrumentation. Flow reversal can also occur due to alternated hemodynamics in the setting of UE AVF or due to extrinsic compression from a variety of factors, both benign and malignant. It is important to recognize flow reversal and to determine its cause as it may have significant clinical implications.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Vikram Dogra is on the Editorial Board of the Journal.
References
- Reversed flow in the left internal jugular vein on time-of-flight MRA as a sign of innominate vein compression syndrome. Clin Radiol. 2007;62:185-8.
- [CrossRef] [PubMed] [Google Scholar]
- Superior thyroid artery pseudoaneurysm and arteriovenous fistula following attempted internal jugular venous access and its management. Indian J Radiol Imaging. 2015;25:15-7.
- [CrossRef] [PubMed] [Google Scholar]
- Jugular venous reflux affects ocular venous system in transient monocular blindness. Cerebrovasc Dis. 2010;29:122-9.
- [CrossRef] [PubMed] [Google Scholar]
- Etiologic considerations in superior vena cava syndrome. Mayo Clin Proc. 1981;56:407-13.
- [Google Scholar]
- Reversed internal jugular vein flow as a sign of brachiocephalic vein obstruction. Australas J Ultrasound Med. 2009;12:39-41.
- [CrossRef] [PubMed] [Google Scholar]
- Color doppler sonographic finding of retrograde jugular venous flow as a sign of innominate vein occlusion. J Clin Ultrasound. 2002;30:392-8.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thrombosis and stenosis after implantation of pacemakers and defibrillators. J Interv Card Electrophysiol. 2005;13:9-19.
- [CrossRef] [PubMed] [Google Scholar]
- Endocranial venous collateral flow in unilateral innominate vein occlusion. Radiology. 1973;107:353-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vector flow imaging techniques: An innovative ultrasonographic technique for the study of blood flow. J Clin Ultrasound. 2017;45:582-8.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound vector flow imaging-could be a new tool in evaluation of arteriovenous fistulas for hemodialysis? J Vasc Access. 2017;18:284-9.
- [CrossRef] [PubMed] [Google Scholar]
- Walker HK, Hurst JW, eds. A Clinical Methods: The History, Physical, and Laboratory Examinations (3rd ed.). Boston: Butterworths; 1990.
- Venous doppler sonography of the extremities: A window to pathology of the thorax, abdomen, and pelvis. AJR Am J Roentgenol. 2009;193:1446-51.
- [CrossRef] [PubMed] [Google Scholar]
- Sonography of the upper extremity and jugular veins. AJR Am J Roentgenol. 1993;160:957-62.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of central venous catheter placement on upper extremity duplex US findings. J Vasc Interv Radiol. 1993;4:399-404.
- [CrossRef] [Google Scholar]
- Color doppler sonography of the thoracic inlet veins. Radiographics. 1995;15:1357-71.
- [CrossRef] [PubMed] [Google Scholar]
- Radiation-induced fibrosis: Mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141:1985-94.
- [CrossRef] [PubMed] [Google Scholar]
- Intracardiac thrombus, superior vena cava syndrome, and pulmonary embolism in a patient with behçet’s disease: A case report and literature review. Heart Vessels. 2007;22:278-83.
- [CrossRef] [PubMed] [Google Scholar]
- Central vein stenosis: Current concepts. Adv Chronic Kidney Dis. 2009;16:360-70.
- [CrossRef] [PubMed] [Google Scholar]
- Angioplasty and stenting of a jugular-carotid fistula resulting from the inadvertent placement of a hemodialysis catheter: Case report and review of literature. Semin Dial. 2012;25:460-3.
- [CrossRef] [PubMed] [Google Scholar]
- Review of evolving etiologies, implications and treatment strategies for the superior vena cava syndrome. Springerplus. 2016;5:229.
- [CrossRef] [PubMed] [Google Scholar]
- Radio-anatomy of the superior vena cava syndrome and therapeutic orientations. Diagn Interv Imaging. 2012;93:569-77.
- [CrossRef] [PubMed] [Google Scholar]
- Subarachnoid hemorrhage after transient global amnesia caused by cerebral venous congestion: Case report. BMC Neurol. 2018;18:36.
- [CrossRef] [PubMed] [Google Scholar]
- Internal jugular, subclavian, and axillary deep venous thrombosis and the risk of pulmonary embolism. Vascular. 2008;16:73-9.
- [CrossRef] [PubMed] [Google Scholar]
- Jugular valve incompetence in transient global amnesia. A problem revisited. J Neuroimaging. 2014;24:479-83.
- [CrossRef] [PubMed] [Google Scholar]
- Cerebral venous thrombosis originating from internal jugular vein outflow impairment: A case report. Medicine (Baltimore). 2017;96:e8975.
- [CrossRef] [PubMed] [Google Scholar]
- Acute internal jugular vein thrombosis associated with pseudoabscess of the retropharyngeal space. AJNR Am J Neuroradiol. 1995;16:892-6.
- [Google Scholar]
- Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-52.
- [CrossRef] [PubMed] [Google Scholar]
- Catheter-directed thrombolysis for treatment of deep venous thrombosis in the upper extremities. Cardiovasc Intervent Radiol. 2009;32:980-7.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular treatment combined with stratified surgery is effective in the management of venous thoracic outlet syndrome complications: A long term ultrasound follow-up study in patients with thrombotic events due to venous thoracic outlet syndrome. Heart Vessels. 2011;26:616-21.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of extrinsic compression of the internal jugular vein in unselected patients undergoing CT angiography. AJNR Am J Neuroradiol. 2012;33:1247-50.
- [CrossRef] [PubMed] [Google Scholar]
- Venous collateral circulation of the extracranial cerebrospinal outflow routes. Curr Neurovasc Res. 2009;6:204-12.
- [CrossRef] [PubMed] [Google Scholar]
- Where there is blood, there is a way: Unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics. 2010;30:67-78.
- [CrossRef] [PubMed] [Google Scholar]
- Superior vena cava obstruction evaluation with MDCT. AJR Am J Roentgenol. 2010;194:W336-46.
- [CrossRef] [PubMed] [Google Scholar]







